Pyeloplasty techniques using minimally invasive surgery (MIS) in pediatric patients
Introduction
Hydronephrosis is the most common urological abnormality identified by prenatal or infant ultrasound, occurring as often as 1% (1). This is now the most common presentation of ureteropelvic junction (UPJ) obstruction. The most common etiology for infant hydronephrosis is UPJ obstruction which accounts for approximately 2.5/100,000 of the hospitalization per year (2-4). The majority of the hospitalizations occur in children less than 3 years old (5). The incidence of UPJ obstruction in those found to have hydronephrosis in infancy is 10–30% (6); this incidence increases in children with other urological abnormalities including horseshoe kidney, in which approximately 17% of have UPJ obstruction (7). The incidence of a crossing vessel causing extrinsic compression on the UPJ has been shown to vary from 15% to 52% (8,9). The variation in the etiology of the obstruction has led to an evolution of repairs to correct the stricture and restore or preserve renal function.
In order to evaluate the current status of minimally invasive surgery (MIS) approach to pyeloplasty, we analyzed the papers presented in literature using Medline on this topic.
Methods
Since 1939, when Foley introduced the Y-V technique, several techniques have been described, but the “gold standard” for UPJ obstruction has become the Anderson-Hynes dismembered pyeleoplasty (10). It allows to remove the entire abnormal tissue, to transpose a crossing vessel if indicated, and multiple approach to the UPJ especially it can be performed using MIS.
The first laparoscopic pyeloplasty (LP) was described by Kavoussi et al. in 1993, they used Anderson-Hynes technique (7) on a young female (24 years old), in 1995 this technique was applied on a child (11,12). In this technique the patient is positioned laterally, with the surgeon and assistant standing on the same side; in younger children 3 mm instruments could be insert with or without trocars. The kidney is exposed by detaching the colon along the avascular line of Toldt. This approach allows the colon to fall medially, exposing the kidney. The renal pelvis was mobilized to achieve sufficient freedom for a tension-free anastomosis. The stay sutures were inserted through the abdominal wall and were placed in ureter side and renal pelvis side. After transecting the pelvis 1-cm proximal to the PUJ, the ureter was spatulated below the stenosis. Another stay suture was inserted through the abdominal wall and was placed in each side to expose the PUJ before starting the anastomosis. The posterior wall was anastomosed first using a running 5-0 PDS suture. When the posterior wall was finished, a JJ stent was placed percutaneously through the abdominal wall by Angiocath and inserted in the PUJ by laparoscopy. The anterior wall was then anastomosed in a similar way. Patients remain on antibiotic prophylaxis until the stent is removed as an outpatient procedure 6 weeks postoperatively (13,14).
Laparoscopy has provided the benefit of better magnification, but this approach does come with a steep learning curve in suturing techniques and tissue manipulation leading to longer operative times (15,16). The suture techniques prove to be especially difficult in children due to smaller tissue and limited abdominal space available for instrument manipulation.
As for the open approach some surgeons prefer the retroperitoneal approach. The retroperitoneal approach was used as previously described (17). Briefly, the patient is placed laterally and retroperitoneal access achieved through the first trocar incision 15 mm long and 1 cm from the lower border of the tip of the 12th rib. Gerota’s fascia is approached by a muscle-splitting blunt dissection then opened under direct vision and the first trocar (5 or 10 mm) introduced directly inside it. A working space is created by gas insufflation dissection, and the first trocar fixed with a purse-string suture applied around the deep fascia, to ensure an airtight seal. A 5- or 10-mm 0 degrees telescope is inserted through the first trocar. A second 3-mm trocar is inserted posteriorly near the costovertebral angle, while the third (3 mm) is inserted 1 cm above the top of the iliac crest at the anterior axillary line. To avoid transperitoneal insertion of this trocar, the working space is fully developed and the deep surface of the anterior wall muscles identified before the trocar is inserted. The insufflation pressure is <12 mmHg and the flow rate of CO2 is progressively increased from 1 to 3 L/min. The kidney is approached posteriorly and the renal pelvis first identified. The PUJ is identified and minimally dissection used to free the PUJ from connective tissue; small vessels are divided after bipolar electrocoagulation. If needed, a fourth trocar (3 mm) is inserted lateral to the lumbosacral muscles near the iliac crest. However, in the last six patients we did not use a fourth trocar. A stay suture of 5/0 polydioxanone is placed for traction at the PUJ. The anterior surface of the PUJ is cleared to identify any polar crossing vessels. The renal pelvis is partly divided by scissors at the most dependent part, when light traction on the stay suture is helpful for manipulating the PUJ. Maintaining the traction, the ureter is partly divided and incised vertically for spatulation. The traction suture helps to mobilize the ureter so that the scissors can be in the axis of the ureter. The anterior surface of the kidney is left adherent to the peritoneum so that the kidney is retracted medially with no need for individual kidney retraction. The pelvi-ureteric anastomosis begins using 6/0 polydioxanone sutures and a tapered 3/8 circular needle. The first suture is placed from the most dependent portion of the pelvis to the most inferior point or vertex of the ureteric spatulation. The suture is tied using the intracorporeal technique with the knots placed outside the lumen. The same suture is used on the anterior wall of the anastomosis. In the initial phase of the study an interrupted anastomosis was made but for the last few cases we adapted the technique and used a running suture. The PUJ is maintained on traction and the suture line stabilized. A 4.7 F polyurethane JJ stent was inserted through the suture line to the bladder at the end of the anterior layer reconstruction, through trocar No. 3. Fluoroscopy was used to assess the placement of the JJ stent in the urinary tract. The pelvis is trimmed if needed. The PUJ and the trimmed part of the pelvis remain undismembered and are removed only after the last suture is placed, thus maintaining stability and decreasing tension on the suture line. The stent remained indwelling for 4–6 weeks. Perirenal suction drainage was used in the beginning, but in the last eight cases no perirenal drainage was placed. A Foley catheter is left in situ 24 h after surgery in all patients. Prophylactic antibiotics (third generation cephalosporin) were routinely prescribed (18). Also a hybrid technique, one trocar assisted pyeloplasty (OTAP), described for the first time in 2004 by El Ghoary has been codificated. The renal pelvis was anteriorly reached using a 10 mm operative telescope via a flank 12 mm incision through a retroperitoneal approach. The UPJ was exteriorized and a dismembered pyeloplasty performed also for the crossing vessel (19,20).
Results
Analyzing the international literature there is no evidence if an approach is better than the other one. A study by Steyaert and Valla underline how both approaches in MIS urology show the same results (21). Just one study to date by Badawy et al. has compared the two approaches in a prospective randomized design. In this study both approaches show good results and seem safe and effective. The group suggests transperitoneal approach which seems to allow a shorter operative time and hospitalization, however, the author underlines that transmesocolic approach could allow a shorter operative time also for this kind of technique (22). In spite of this, urologists for adults suggest transperitoneal route during learning curve for laparoscopy because of longer operative time for access to the operative field and suturing (23). They also show the same outcomes for both approaches in experienced surgeons, but in several series a longer operative time is signaled (24-26).
The research on transperitoneal way to approach UPJ obstruction shows several studies. The first series by Tan et al. in 1999 shows its feasibility and safeness in children (27). All the other series confirmed these good results. They are summarized in table below (Table 1) and compared with that have been treated by a retroperitoneal approach (13,14,18,27-31). In OTAP series by Lima et al. the average operative time of 139 minutes, a success rate of 87.5% and two conversions were reported (32). This hybrid technique should be considered as a good step for surgeon making transition to LP (33).
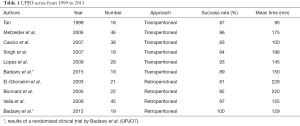
Full table
Discussion
In the last 20 years laparoscopic Anderson-Hynes pyeloplasty in children has become an acceptable alternative to the open procedure, with results approaching those of conventional open surgery. Laparoscopy reproduces the advantages of open pyeloplasty, including the mucosa-to-mucosa anastomosis, and excision of any redundant renal pelvis and diseased ureter. The procedure has gained in popularity and more recent series have shown a success rate of greater than 95%. LP in children has followed the same evolution as nephrectomy. The procedure was first described through a transperitoneal approach. Tan reported the first pediatric series of transperitoneal laparoscopic dismembered pyeloplasty in 18 children with variable age from 3 months to 15 years old (27).
Some studies underline the relevance of timing for surgery and confined this technique to children older than 6 months (13,29) for both approaches, in these patients could be helpful OTAP technique. Lima et al. reported an average age of 5.6 months and a success rate of 91% in line with literature as for open technique as for MIS (32).
Transperitoneal route is preferred because it maximizes the internal working space and is far more ergonomic for intracorporeal suturing. Given that the ureteropelvic anastomosis is the most critical part of this procedure, we believe that the ergonomics of suturing should not be compromised for the sake of adhering to an extra peritoneal route, especially since there is no evidence to suggest an advantage (13). This is also underlined by the average time of all the series considered as shown in Table 1. In spite of this some centers prefer retroperitoneal approach, because they feel more confident with this technique and there are no other differences in terms of complications and conversions (30).
Urine leakage has not been reported in series cited, but this complication would be better tolerated in the retroperitoneal space than in the intraperitoneal cavity. In literature a horseshoe kidney is an anatomical indication for the transperitoneal approach.
Yeung et al. reported their initial experience with retroperitoneal dismembered pyeloplasty in 13 patients, of whom 1 required open conversion. Average operative time was 143 minutes (range, 103 to 235 minutes). The longer time needed for the retroperitoneal approach is probably related to the limited working space, which renders suturing more difficult (34).
In their study, Shoma et al. pointed out that the presence of a crossing vessel is significantly related to increased operative times, as anastomosis is more difficult, especially in the retroperitoneal approach (24).
El-Ghoneimi et al. encountered crossing vessels in nine children: the anterior transposition of the PUJ was performed and no significant prolongation of operative time was reported (18).
There were no disadvantages attributable to the transabdominal approach in other series.
We believe that the exposition of the renal pelvis, the ureter and, occasionally, the aberrant pole vessels was excellent, and the working space for suturing and knotting was adequate, including in children younger than 1 year as suggested in work by Metzelder (28). Also Valla et al. underline that the most important point in utilizing the retroperitoneoscopic approach is patient size. Small patients, <6 kg, in our experience, have limited retroperitoneal space making retroperitoneal suturing particularly challenging (30).
Previous retroperitoneal surgery and previous percutaneous nephrostomy for drainage are usually considered to be contra-indications for the retroperitoneoscopic approach (30).
In terms of learning curve all the cited authors confirm the importance of a deep laparoscopic training epecially in suturing and knotting.
In conclusion laparoscopic reconstructive urology has evolved rapidly in the past 20 years and will continue to do so. Greatest experience has been gained in LP and the many large series published have demonstrated the safety and efficacy of this procedure in the management of UPJ obstruction in children. Therefore, the laparoscopic approach should be considered the “gold standard” for the management of such patients. Because of few comparative studies, the choice between the transperitoneal and retroperitoneal approach is quite subjective and depends on the experience and preference of the individual surgeon.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lee RS, Borer JG. Perinatal urology. In:McDougal WS, Wein AJ, Kavoussi LR. editors. Campbell-Walsh Urology. Ch 114. 10th edition. Saunders, 2011:3048-66.
- Chandrasekharam VV, Srinivas M, Bal CS, et al. Functional outcome after pyeloplasty for unilateral symptomatic hydronephrosis. Pediatr Surg Int 2001;17:524-7. [Crossref] [PubMed]
- Kato Y, Yamataka A, Okazaki T, et al. Surgical treatment and outcome of mega-hydronephrosis due to pelviureteric junction stenosis. Pediatr Surg Int 2006;22:911-3. [Crossref] [PubMed]
- Knoedler J, Han L, Granberg C, et al. Population-based comparison of laparoscopic and open pyeloplasty in paediatric pelvi-ureteric junction obstruction. BJU Int 2013;111:1141-7. [Crossref] [PubMed]
- Schulam P. Chapter 9. Ureteropelvic junction obstruction. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office, 2007:323-32.
- Garg RK, Menon P, Narasimha Rao KL, et al. Pyeloplasty for hydronephrosis: Issues of double J stent versus nephrostomy tube as drainage technique. J Indian Assoc Pediatr Surg 2015;20:32-6. [Crossref] [PubMed]
- Kavoussi LR, Peters CA. Laparoscopic pyeloplasty. J Urol 1993;150:1891-4. [PubMed]
- Lowe FC, Marshall FF. Ureteropelvic junction obstruction in adults. Urology 1984;23:331-5. [Crossref] [PubMed]
- Stephens FD. Ureterovascular hydronephrosis and the "aberrant" renal vessels. J Urol 1982;128:984-7. [PubMed]
- Seo IY, Oh TH, Lee JW. Long-term follow-up results of laparoscopic pyeloplasty. Korean J Urol 2014;55:656-9. [Crossref] [PubMed]
- Szavay PO, Luithle T, Seitz G, et al. Functional outcome after laparoscopic dismembered pyeloplasty in children. J Pediatr Urol 2010;6:359-63. [Crossref] [PubMed]
- Peters CA, Schlussel RN, Retik AB. Pediatric laparoscopic dismembered pyeloplasty. J Urol 1995;153:1962-5. [Crossref] [PubMed]
- Cascio S, Tien A, Chee W, et al. Laparoscopic dismembered pyeloplasty in children younger than 2 years. J Urol 2007;177:335-8. [Crossref] [PubMed]
- Lopez M, Guye E, Varlet F. Laparoscopic pyeloplasty for repair of pelvi-ureteric junction obstruction in children. J Pediatr Urol 2009;5:25-9. [Crossref] [PubMed]
- Ravish IR, Nerli RB, Reddy MN, et al. Laparoscopic pyeloplasty compared with open pyeloplasty in children. J Endourol 2007;21:897-902. [Crossref] [PubMed]
- Bansal D, Cost NG, Bean CM. Infant robot-assisted laparoscopic upper urinary tract reconstructive surgery. J Pediatr Urol 2014;10:869-74. [Crossref] [PubMed]
- El-Ghoneimi A, Valla JS, Steyaert H, et al. Laparoscopic renal surgery via a retroperitoneal approach in children. J Urol 1998;160:1138-41. [Crossref] [PubMed]
- El-Ghoneimi A, Farhat W, Bolduc S, et al. Laparoscopic dismembered pyeloplasty by a retroperitoneal approach in children. BJU Int 2003;92:104-8; discussion 108. [Crossref] [PubMed]
- Lima M, Tursini S, Ruggeri G, et al. One trocar assisted pyeloplasty (OTAP): initial experience and codification of a technique. Pediatr Med Chir 2007;29:108-11. [PubMed]
- Scuderi MG, Arena S, Di Benedetto V. One-trocar-assisted pyeloplasty. J Laparoendosc Adv Surg Tech A 2011;21:651-4. [Crossref] [PubMed]
- Steyaert H, Valla JS. Minimally invasive urologic surgery in children: an overview of what can be done. Eur J Pediatr Surg 2005;15:307-13. [Crossref] [PubMed]
- Badawy H, Zoaier A, Ghoneim T, et al. Transperitoneal versus retroperitoneal laparoscopic pyeloplasty in children: Randomized clinical trial. J Pediatr Urol 2015;11:122.e1-6. [Crossref] [PubMed]
- Zhu H, Shen C, Li X, et al. Laparoscopic pyeloplasty: a comparison between the transperitoneal and retroperitoneal approach during the learning curve. Urol Int 2013;90:130-5. [Crossref] [PubMed]
- Shoma AM, El Nahas AR, Bazeed MA. Laparoscopic pyeloplasty: a prospective randomized comparison between the transperitoneal approach and retroperitoneoscopy. J Urol 2007;178:2020-4; discussion 2024. [Crossref] [PubMed]
- Abuanz S, Gamé X, Roche JB, et al. Laparoscopic pyeloplasty: comparison between retroperitoneoscopic and transperitoneal approach. Urology 2010;76:877-81. [Crossref] [PubMed]
- Wu Y, Dong Q, Han P, et al. Meta-analysis of transperitoneal versus retroperitoneal approaches of laparoscopic pyeloplasty for ureteropelvic junction obstruction. J Laparoendosc Adv Surg Tech A 2012;22:658-62. [Crossref] [PubMed]
- Tan HL. Laparoscopic Anderson-Hynes dismembered pyeloplasty in children. J Urol 1999;162:1045-7; discussion 1048. [Crossref] [PubMed]
- Metzelder ML, Schier F, Petersen C, et al. Laparoscopic transabdominal pyeloplasty in children is feasible irrespective of age. J Urol 2006;175:688-91. [Crossref] [PubMed]
- Bonnard A, Fouquet V, Carricaburu E, et al. Retroperitoneal laparoscopic versus open pyeloplasty in children. J Urol 2005;173:1710-3; discussion 1713.
- Valla JS, Breaud J, Griffin SJ, et al. Retroperitoneoscopic vs open dismembered pyeloplasty for ureteropelvic junction obstruction in children. J Pediatr Urol 2009;5:368-73. [Crossref] [PubMed]
- Singh H, Ganpule A, Malhotra V, et al. Transperitoneal laparoscopic pyeloplasty in children. J Endourol 2007;21:1461-6. [Crossref] [PubMed]
- Lima M, Ruggeri G, Messina P, et al. One-trocar-assisted pyeloplasty in children: an 8-year single institution experience. Eur J Pediatr Surg 2015;25:262-8. [PubMed]
- Sukumar S, Nair B, Sanjeevan KV, et al. Laparoscopic assisted dismembered pyeloplasty in children: intermediate results. Pediatr Surg Int 2008;24:403-6. [Crossref] [PubMed]
- Yeung CK, Tam YH, Sihoe JD, et al. Retroperitoneoscopic dismembered pyeloplasty for pelvi-ureteric junction obstruction in infants and children. BJU Int 2001;87:509-13. [Crossref] [PubMed]


