Laparoscopic approach for gonadectomy in pediatric patients with intersex disorders
Definition
Disorders of sex development (DSD) are rare congenital anomalies with atypical chromosome, gonadal or anatomical sex organ development. DSD is a generic term introduced in 2005 (Chicago consensus conference) that applies to different congenital conditions, many of which are characterized by the unusual appearance of external genitalia and/or atypically developed gonads with potential negative consequences on psychosexual development, fertility and cancer risk. The same consensus meeting tried classifying these complex conditions according to their underlying chromosomal profiles (1,2).
DSD is currently classified into three main groups (Table 1):

Full table
- The 46,XX DSD patients are individuals who are genetically female, most commonly due to Congenital Adrenal Hyperplasia (CAH), and present with an overdeveloped genital tubercle (clitoris), no vaginal connection to the perineum and enlarged and merged genital folds. The internal genitalia are female and usually normal;
- The 46,XY DSD patients are genetically male and constitute a more heterogeneous group, representing a spectrum from normal appearing females to males with hypospadias and infertility. These patients may have underdevelopment of the genital tubercle (hypospadias and/or micropenis) with or without undescended gonads, with or without feminine remnants (mullerian structures). Within this group are patients with dysfunctional gonads (gonadal dysgenesis), impaired steroidogenesis (17 beta hydroxysteroid dehydrogenase deficit), dysfunctional central hormonal control, and dysfunctional target tissues (androgen insensitivity; 5 alpha reductase deficiency);
- The chromosomal abnormalities or mosaicisms are mostly represented by the 45,X0/46,XY individuals (mixed gonadal dysgenesis). This group comprises the ovo-testicular DSD. These two last groups of patients bear both female and male genetic and anatomic elements. They typically have asymmetric genitalia with one side more masculine and the other side more feminine. The gonads can be testis, ovary or both, or dysgenetic gonads with a high risk for gonadal tumor development later in life.
Investigation and management of DSD
Optimal clinical management of individuals with DSD should comprise the following aspects: (I) gender assignment must be avoided prior to expert evaluation in newborns; (II) evaluation and long-term management must be performed at a center with an experienced multidisciplinary team; (III) all individuals should receive a gender assignment; (IV) open communication with patients and families is essential and participation in decision-making is encouraged; (V) patient and family concerns should be respected and addressed in strict confidence (3).
A key point to emphasize is that the DSD child has the potential to become a well-adjusted, functional member of society. It should be explained to the parents that the best course of action may not initially be clear, but the health care team will work with the family to reach the best possible set of decisions in the circumstances. The health care team should discuss with the parents what information to share in the early stages with family members and friends.
Diagnostic context
The context of DSD diagnosis can be described in three periods of life:
Prenatally, discordance between the ultrasound appearance of the foetus’s genitalia and the karyotype can suggest a possible DSD.
A common presentation is at birth with a child born with unusual genitalia. In some complex cases, assignment of the child’s gender can be delayed by the medical team until the diagnostic process is completed. Assigning a gender based upon anatomical (phenotype) and biological criteria without certainty of the individual’s ultimate gender identity is a major challenge which is at the core of some of the criticisms directed at the medical team. The diagnosis can occur later in life, as in an individual raised as female who undergoes surgery for an inguinal hernia in which testis are found in whom primary amenorrhea is diagnosed (complete androgen insensitivity syndrome/Morris syndrome) or a girl who presents signs of virilization at puberty (5 alpha reductase; 17 beta hydroxysteroid reductase).
Alternatively, an individual raised as male may present with gynecomastia or infertility. In this group of patients, the gender identity is usually well established (1,2).
The multidisciplinary team
Optimal care for children with DSD requires an experienced multidisciplinary team that is generally found in tertiary care centers. Ideally, the team includes pediatric subspecialists in endocrinology, surgery and/or urology, psychology/psychiatry, gynecology, genetics, neonatology and, if available, social work, nursing and medical ethics (4).
Ongoing communication with the family primary care physician is essential (5). The team has a responsibility to educate other health care staff in the appropriate initial management of affected newborns and their families.
For new DSD patients, the team should develop a plan for clinical management with respect to diagnosis, gender assignment and treatment options before making any recommendations. Ideally, discussions with the family are conducted by one professional with appropriate communication skills (6). Transitional care should be organized with the multidisciplinary team operating in an environment comprising specialists with experience in both pediatric and adult practice. Support groups can have an important role in the delivery of care to DSD patients and their families (7).
Clinical evaluation
A family and prenatal history, a general physical examination with attention to any associated dysmorphic features and an assessment of the genital anatomy in comparison to published norms needs to be recorded (2). Criteria that suggest DSD include: (I) overt genital ambiguity (e.g., cloacal exstrophy); (II) apparent female genitalia with an enlarged clitoris, posterior labial fusion, or an inguinal/labial mass; (III) apparent male genitalia with bilateral undescended testes, micropenis, isolated perineal hypospadias or mild hypospadias with undescended testis; (IV) a family history of DSD such as complete androgen insensitivity syndrome (CAIS/Morris syndrome); (V) a discordance between genital appearance and a prenatal karyotype. Most causes of DSD are recognized in the neonatal period; later presentations in older children and young adults include: (I) previously unrecognized genital ambiguity; (II) delayed or incomplete puberty; (III) virilization in a female; (IV) primary amenorrhea; (V) breast development in a male; (VI) gross and occasionally cyclic hematuria in a male.
Diagnostic evaluation
A specific molecular diagnosis is identified in only about 20% of cases of DSD. The majority of virilized 46, XX infants will have congenital adrenal hyperplasia (CAH). In contrast, only 50% of 46 XY children with DSD will receive a definitive diagnosis (8,9).
Diagnostic algorithms do exist. Some tests, such as imaging by ultrasound, are operator dependent. Hormone measurements need to be interpreted in relation to the specific assay characteristics, and to normal values for gestational and chronological age. In some cases serial measurements may be needed.
First-line testing in newborns includes: karyotyping with X and Y-specific probe detection (even when prenatal karyotype is available), imaging (abdomino-pelvic ultrasound), measurement of 17-hydroxyprogesterone, testosterone, gonadotropins, anti-Mullerian hormone, serum electrolytes and urine analysis. The results of these investigations are generally available within 48 hours and will be sufficient for making a working diagnosis. Decision making algorithms are available to guide further investigation (10). These include hCG and ACTH stimulation tests to assess testicular and adrenal steroid biosynthesis, urinary steroid analysis by GC mass spectroscopy, imaging studies and biopsies of gonadal material. Some gene analyses are performed in clinical service laboratories.
In regard to imaging exams, a combination of ultrasound and magnetic resonance imaging (MRI) to determine the exact location of the gonads allows appropriate surgical planning in many cases.
What does surgery entail?
The surgeon has a responsibility to outline the surgical sequence and subsequent consequences from infancy to adulthood. Only surgeons with expertise in the care of children and specific training in the surgery of DSD should perform these procedures (1).
Feminization procedures include the opening of the vaginal cavity to the perineum as in CAH or, androgen insensitivity syndromes, steroidogenesis deficits, enlargement or creation of a vagina either by dilatation or by substitution (bowel, peritoneum), the possible reduction of the genital tubercle for clitoral hypertrophy with nerve preservation, and reconstruction of the perineum.
Masculinization procedures include hypospadias surgery or, in rare cases, phalloplasty.
Ovaries are usually preserved unless associated with dysgenetic testicular tissue which may carry a risk of malignancy.
Testes are either brought down in boys or removed if dysgenetic with tumor risk or in complete androgen insensitivity syndrome (CAIS—Morris syndrome) or 5 alpha reductase deficiency.
Testicular prostheses can be inserted at puberty at the patient’s request.
Mullerian remnants can also be removed in boys if they cause urological (infection, dysuria) or gynecological (menstruation) symptoms.
The role of laparoscopy for gonadectomy in DSD patients
Gonadectomy is indicated in children with intersex disorders harboring Y chromosomal material as a result of the malignant potential of their gonads (11). A variety of tumors have been found to be frequently associated, the most common being the gonadoblastoma, which was found to a significant degree in a large case series of women with a Y chromosome (12).
Traditional surgery for those with intersex disorders is a laparotomy and bilateral gonadectomy. However, with increasing experience, laparoscopic evaluation of the pelvic structures and removal of intra-abdominal gonads has become a part of the management of intersex disorders. It is more acceptable to the patient than laparotomy. The widespread use of laparoscopic gonadectomy in adults has prompted its use in the treatment of children and adolescents.
Laparoscopy is well suited in this age group conferring advantages regarding post-operative recovery, length of hospitalisation and improved cosmesis. These advantages may reduce psychological trauma—a significant factor in intersex children (13).
Timing of surgery
Appropriate timing of gonadectomy hinges on an understanding about the different intersex disorders. The chances of neoplasia and virilisation in the dysgenetic or streak gonads found in some conditions at puberty are estimated at 30% (11). It has been shown that tumors can arise as early as six months (11).
In complete androgen insensitivity syndrome (CAIS-Morris syndrome), the gonads are essentially normal gonads with an approximately 5% risk of tumor disease. With complete androgen insensitivity syndrome, there is also no risk of virilisation at puberty as a result of lack of androgen response and so a prophylactic gonadectomy relatively late, at age 16 to 18, to allow the completion of secondary sexual development may be advocated.
The testes in patients with CAIS and those with partial androgen insensitivity syndrome (PAIS), raised female, should be removed to prevent malignancy in adulthood (14). The availability of estrogen replacement therapy allows for the option of early removal at the time of diagnosis which also takes care of the associated hernia, psychological problems with the presence of testes and the malignancy risk. Parental choice allows deferment until adolescence, recognizing that the earliest reported malignancy in CAIS is at 14 years of age (15).
The streak gonad in a patient with mixed gonadal dysgenesis (MGD) raised male should be removed laparoscopically in early childhood (14).
Bilateral gonadectomy is performed in early childhood in females (bilateral streak gonads) with gonadal dysgenesis and Y chromosome material.
In patients with androgen biosynthetic defects raised female, gonadectomy should be performed before puberty.
A scrotal testis in patients with gonadal dysgenesis is at risk for malignancy. Current recommendations are testicular biopsy at puberty seeking signs of the pre-malignant lesion termed carcinoma-in-situ or undifferentiated intratubular germ cell neoplasia. If positive, the option is sperm banking before treatment with local low dose radiotherapy which is curative (16).
Risk of gonadal tumors
Interpretation of the literature is hampered by unclear terminology and effects of normal cell maturation delay (17-19). The highest tumor risk is found in TSBY (testis-specific protein Y encoded) positive gonadal dysgenesis and PAIS with intra-abdominal gonads, while the lowest risk (<5%) is found in ovotestis (20) and CAIS (18,21). In the current literature, 3 categories of risk have been identified (1):
- High risk: complete gonadal dysgenesis; Partial gonadal dysgenesis with non-scrotal gonad; Frazier syndrome; Partial androgen insensitivity syndrome (PAIS) with non-scrotal gonad; Denys—Drash syndrome;
- Intermediate risk: 17 β-hydroxysteroid dehydrogenase deficiency; partial androgen insensitivity syndrome (PAIS) with scrotal gonad; Y-chromosome Turner syndrome;
- Low risk: complete androgen insensitivity syndrome (CAIS/Morris syndrome); ovotesticular DSD; Turner syndrome lacking a Y-chromosome in their karyotype; Persistent Müllerian duct syndrome.
Surgical technique
The patients have a general anaesthetic with endotracheal and nasogastric intubation. The video monitor, insufflator and light source are positioned at the foot of the patient. A carbon dioxide pneumoperitoneum is created using the open laparoscopy technique under vision through an umbilical incision. A 3- or 4-port laparoscopy is performed.
The primary10-mm port is inserted through the umbilicus for the 10-mm 0° telescope, and 2 or 3 secondary 3- or 5-mm pelvic ports are inserted for working instruments. Laparoscopic inspection of the pelvis is carried out to determine the presence of gonads and to inspect the pelvic organs. In some cases when the gonads are not easily identified, the gonadal vessels are identified and followed down. The gonads are removed after determination of the course of the ureters and the fallopian tube; they are also excised when the gonads appear continuous with them. Some special hemostatic devices are used in some cases, to complete the resection or the hemostasis. At the end of the procedure, a 5-mm laparoscope is inserted through 1 of the lateral ports so that the gonads can be extracted through the central umbilical 10-mm port.
Sex steroid replacement
Hypogonadism is common in patients with dysgenetic gonads, defects in sex steroid biosynthesis and resistance to androgens.
The timing of initiation of puberty may vary but this is an occasion that provides an opportunity to discuss the condition and set a foundation for long-term adherence to therapy. Hormonal induction of puberty should attempt to replicate normal pubertal maturation to induce secondary sexual characteristics, a pubertal growth spurt, and optimal bone mineral accumulation, together with psychosocial support for psychosexual maturation (22). Intramuscular depot injections of testosterone esters are commonly used in males; other options include oral testosterone undecanoate and transdermal preparations are also available (23-25). Patients with PAIS may require supraphysiologic doses of testosterone for optimal effect (26). Females with hypogonadism require estrogen supplementation to induce pubertal changes and menses. A progestin is usually added after breakthrough bleeding develops or within 1–2 years of continuous estrogen. There is no evidence that the addition of cyclic progesterone is beneficial in women without a uterus.
Literature analysis
Trying to find the evidence, in this review we performed a literature analysis using PubMed, Cochrane, and Medline databases on all studies published during the last 10 years that described laparoscopic gonadectomy in DSD patients. The following key words were used: “laparoscopic”, “gonadectomy”, “DSD”, “children”, “adolescents”, “intersex disorders”, “intersex patients”.
Searches were also performed using the following limits: clinical trials, randomized controlled trials, multicenter retrospective, prospective studies, and expert opinion. Conference abstracts were excluded because of the limited data presented in them. Publications with evidence of possible overlap were also excluded from this review. Although no language restrictions were imposed initially, the search was limited to studies published in the English language for the full-text review and final analysis. Eligibility criteria included all available studies focused on laparoscopic gonadectomy in DSD patients and with quantitative data on outcome parameters. The pediatric population was defined as younger than 18 years when the patient underwent laparoscopic gonadectomy. After relevant titles were identified, the abstracts of these studies were read to decide if the study was eligible. The full article was retrieved when the information in the title and/or abstract appeared to meet the objective of our review. The authors independently assessed selected studies and tabulated data from each article with a predefined data extraction form. Data regarding the following factors were considered: first author, publication date, study method, participant features, intervention characteristics, definition of complications, and outcome measures.
We recorded 23 studies, but only 10 papers (27-36) were included in the analysis since they responded to the selected inclusion criteria.
Results
Surgical outcome
Our review included 168 patients with various forms of DSD. All patients underwent laparoscopic gonadectomy; average age at surgery was 11.6 years (range, 3–18.3 years).
All procedures were completed laparoscopically. Average operative time was 70 minutes. No conversions to open surgery neither intra-operative complications were reported in all series. Mean length of hospital stay was 1.4 days (range, 1–2 days).
Post-operative complications were reported only in one series (28) and included 1 umbilical port infection [2% (1/50)] and 1 pelvic abscess [2% (1/50)], both treated with antibiotic therapy (grade I Clavien-Dindo).
Incidence of gonadal tumors
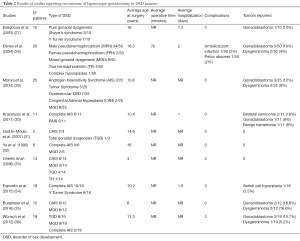
Of the analyzed series, 7/10 reported postoperative diagnosis of gonadal tumors (27-30,34-36). The histopathologic examinations revealed 15 cases of gonadoblastoma, 7 cases of dysgerminoma and 2 cases of seminoma.
Analyzing the single series, the incidence of these tumors varied between 10% and 33%. The majority of malignant tumors were reported in Total Gonadal Dysgenesis (TGD) and Mixed Gonadal Dysgenesis (MGD) (11 cases of gonadoblastoma, 6 cases of dysgerminoma), followed by PAIS (2 cases of seminoma, 1 case of gonadoblastoma), Y Turner syndrome (2 cases of gonadoblastoma), Ovotestis DSD (1 case of gonadoblastoma, 1 case of dysgerminoma), CAIS (1 case of gonadoblastoma).
Pathology revealed also benign anomalies comprising 1 benign amartoma and 1 Sertoli cell hyperplasia in 2 patients with PAIS (30,34). All results are summarized in Table 2.
Full table
Discussion
In DSD patients, the first indication for gonadectomy is to remove the gonad, which contradicts the assigned social gender of the patient (29). The second indication for gonadectomy is to remove the dysgenetic gonad in patients with the presence of a Y chromosome (29). In fact, patients with specific forms of disorders of sex development harboring Y-chromosomal material have an increased risk for the development of cancers originating from the germ-cell lineage, also known as germ-cell tumors (37). The risk of gonadal neoplasia is not confined to patients with a 46,XY karyotype but extends to patients with any mosaic karyotype containing a Y-chromosome or the SRY antigen (11). We found in the literature that gonadoblastoma and seminomas are the most frequently occurring malignant tumors in gonadal intersex disorders (34).
Benign tumors such as hamartomas, Sertoli cell adenomas, and, rarely, Leydig-cell tumors have been reported in association with Morris syndrome/CAIS. Current recommendations for CAIS patients are to retain the cryptorchid gonads through puberty to receive benefit from their hormone production; enhance bone maturation; and to allow the completion of secondary sexual development. However, there is a high tendency to perform an early gonadectomy whenever a diagnosis of PAIS is made, in whom broad spectrum of defect mutations and genetic mechanisms underlying this syndrome may hide significant risk for malignancy (30).
To our knowledge, tests or markers that permit one to easily distinguish (pre)malignant from normal germ cells are not available. By reviewing images (computed tomography/magnetic resonance imaging) of these tumors, it is difficult to exclude possible neoplastic nature and preclude surgery for definitive diagnosis (30). Dénes et al. reported a prevalence rate of tumoral disease of 14% but noted that only 3 of the malignant gonads had macroscopic abnormalities suggesting tumor (28). “In-situ” malignant lesions can be excluded only with histopathologic examination of the gonads. For these reasons, gonadectomy is considered the modality of choice for the treatment of these patients.
However, the indication for a laparoscopic abdominal gonadal tumor is a matter of debate in gynecologic surgery because of the risks of port site metastasis (38). Recent reviews proposed that most port site metastases occurred in cases with advanced disease or intra-abdominal spillage (39). Since gonadectomy for DSD patients is indicated as prophylactic surgery due to the malignant potential, this kind of surgery would be indicated, unless a gonadal tumor is detected by preoperative assessment (29).
The widespread use of laparoscopic gonadectomy in adults has prompted its use in the treatment of children and adolescents. The classic advantages of laparoscopy include low morbidity, excellent cosmetic results, shorter hospitalization, and rapid return to normal activities. In addition, laparoscopy gives an excellent view of the pelvic structures, including the genital organs (27). Laparoscopic evaluation can also serve as a useful surgical adjunct enabling better visualisation and localisation of the gonads and mullerian ductal remnants as well as the uterus and vaginal components of the urogenital sinus (29).
Another consideration is what should be done with normal mullerian structures.
Consensus Statement on Management of Intersex Disorders in 2006 (2) and the ESPU/SPU standpoint on the surgical management of Disorders of Sex Development (1) stated that ‘In patients with a symptomatic utriculus, removal is best performed laparoscopically’ and that ‘Mullerian remnants can also be removed in boys if they cause urological (infection, dysuria) or gynecological (menstruation) symptoms’, respectively. Recently, the routine resection of Mullerian duct derivatives for patients without any symptom is not performed because literature rarely describes malignancy in Mullerian duct derivatives, with most reports involving persistent Mullerian remnant syndrome (38).
Preservation of the uterus is important in patients with Turner syndrome because there have been reports of pregnancy by ovum donation in this group of women (40). However, with regard to the fallopian tubes, it has been suggested that complete removal of the gonads can be best accomplished by adnexectomy rather than gonadectomy. This may be especially when the gonads are elongated, attenuated and closely approximated to the fallopian tube. It has also been suggested that as intrauterine embryo transfer is the preferred technique during IVF, there is less importance in preserving the fallopian tubes (27).
Surgical management in DSD should also consider options that will facilitate the chances of fertility. In patients with a symptomatic utriculus, removal is best performed laparoscopically to increase the chance of preserving continuity of the vasa deferentia (2). Patients with bilateral ovotestes are potentially fertile from functional ovarian tissue (41). Separation of ovarian and testicular tissue can be technically difficult and should be undertaken, if possible, in early life.
It has been reported that oocyte cryopreservation is a feasible technique in selected post-pubertal female children at risk for premature ovarian failure and infertility as happens in Turner syndrome (42,43). In our review, only 1 series reported ovarian cryopreservation in an attempt to maintain the fertility of patients with Turner syndrome (34).
In all series reported in this review, all laparoscopic surgeries were completed without conversion to open surgery, significant bleeding, or intraoperative complications. In addition, besides laparoscopy having the advantages of being a minimally invasive approach, a more favorable cosmetic result is also an important factor for young patients already involved in complex psychological and social relationships. This is another advantage of laparoscopy for patients with DSD (27,29).
However, debate is still open about indications and timing of gonadectomy. Based on the non trascurabile rate of tumoral risk reported in our review, variable between 10% and 33%, the availability of estrogen replacement therapy to allow secondary sexual development and the performance of cryopreservation to preserve fertility allow for the option of early gonadectomy at the time of diagnosis.
We believe that laparoscopic gonadectomy should be accepted as the treatment of choice in children and adolescents with these rare conditions. It thereby eliminates the risk of malignancies of gonadal origin with the advantages of a minimally invasive procedure, with lower morbidity, quicker postoperative recovery and excellent cosmetic results. This latter aspect is especially important for patients with intersex disorders who need reaffirmation of their body image and self-esteem.
In addition, it should be offered within a specialist unit offering the full range of multidisciplinary medical and psychological support for children and teenagers with these complex conditions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mouriquand P, Caldamone A, Malone P, et al. The ESPU/SPU standpoint on the surgical management of Disorders of Sex Development (DSD). J Pediatr Urol 2014;10:8-10. [Crossref] [PubMed]
- Hughes IA, Houk C, Ahmed SF, et al. Consensus statement on management of intersex disorders. J Pediatr Urol 2006;2:148-62. [Crossref] [PubMed]
- Consortium on the Management of Disorders of Sex Differentiation. Clinical guidelines: for the management of disorders of sex development in childhood. 2006. Available online: http://www.dsdguidelines.org
- Lee PA. A perspective on the approach to the intersex child born with genital ambiguity. J Pediatr Endocrinol Metab 2004;17:133-40. [Crossref] [PubMed]
- American Academy of Pediatrics Council on Children with Disabilities. Care coordination in the medical home: integrating health and related systems of care for children with special health care needs. Pediatrics 2005;116:1238-44. [Crossref] [PubMed]
- Cashman S, Reidy P, Cody K, et al. Developing and measuring progress toward collaborative, integrated, interdisciplinary health care teams. J Interprof Care 2004;18:183-96. [Crossref] [PubMed]
- Warne G. Clinical note: Support groups for CAH and AIS. Endocrinologist 2003;13:175-8. [Crossref]
- Ahmed SF, Cheng A, Dovey L, et al. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J Clin Endocrinol Metab 2000;85:658-65. [PubMed]
- Morel Y, Rey R, Teinturier C, et al. Aetiological diagnosis of male sex ambiguity: a collaborative study. Eur J Pediatr 2002;161:49-59. [Crossref] [PubMed]
- Ogilvy-Stuart AL, Brain CE. Early assessment of ambiguous genitalia. Arch Dis Child 2004;89:401-7. [Crossref] [PubMed]
- Manuel M, Katayama PK, Jones HW Jr. The age of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol 1976;124:293-300. [Crossref] [PubMed]
- Scully RE. Gonadoblastoma. A review of 74 cases. Cancer 1970;25:1340-56. [Crossref] [PubMed]
- LaMontagne LL, Hepworth JT, Cohen F. Effects of surgery type and attention focus on children's coping. Nurs Res 2000;49:245-52. [Crossref] [PubMed]
- Grumbach MM, Hughes IA, Conte FA. Disorders of sex differentiation. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams textbook of endocrinology. 10th ed. Philadelphia (PA): Saunders. 2003:842-1002.
- Hurt WG, Bodurtha JN, McCall JB, et al. Seminoma in pubertal patient with androgen insensitivity syndrome. Am J Obstet Gynecol 1989;161:530-1. [Crossref] [PubMed]
- Rørth M, Rajpert-De Meyts E, Andersson L, et al. Carcinoma in situ in the testis. Scand J Urol Nephrol Suppl 2000.166-86. [Crossref] [PubMed]
- Honecker F, Stoop H, de Krijger RR, et al. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol 2004;203:849-57. [Crossref] [PubMed]
- Cools M, van Aerde K, Kersemaekers AM, et al. Morphological and immunohistochemical differences between gonadal maturation delay and early germ cell neoplasia in patients with undervirilization syndromes. J Clin Endocrinol Metab 2005;90:5295-303. [Crossref] [PubMed]
- Cools M, Honecker F, Stoop H, et al. Maturation delay of germ cells in fetuses with trisomy 21 results in increased risk for the development of testicular germ cell tumors. Hum Pathol 2006;37:101-11. [Crossref] [PubMed]
- Ramani P, Yeung CK, Habeebu SS. Testicular intratubular germ cell neoplasia in children and adolescents with intersex. Am J Surg Pathol 1993;17:1124-33. [Crossref] [PubMed]
- Hannema SE, Scott IS, Rajpert-De Meyts E, et al. Testicular development in the complete androgen insensitivity syndrome. J Pathol 2006;208:518-27. [Crossref] [PubMed]
- Warne GL, Grover S, Zajac JD. Hormonal therapies for individuals with intersex conditions: protocol for use. Treat Endocrinol 2005;4:19-29. [Crossref] [PubMed]
- Rogol AD. New facets of androgen replacement therapy during childhood and adolescence. Expert Opin Pharmacother 2005;6:1319-36. [Crossref] [PubMed]
- Ahmed SF, Tucker P, Mayo A, et al. Randomized, crossover comparison study of the short-term effect of oral testosterone undecanoate and intramuscular testosterone depot on linear growth and serum bone alkaline phosphatase. J Pediatr Endocrinol Metab 2004;17:941-50. [Crossref] [PubMed]
- Mayo A, Macintyre H, Wallace AM, et al. Transdermal testosterone application: pharmacokinetics and effects on pubertal status, short-term growth, and bone turnover. J Clin Endocrinol Metab 2004;89:681-7. [Crossref] [PubMed]
- Weidemann W, Peters B, Romalo G, et al. Response to androgen treatment in a patient with partial androgen insensitivity and a mutation in the deoxyribonucleic acid-binding domain of the androgen receptor. J Clin Endocrinol Metab 1998;83:1173-6. [PubMed]
- Esegbona G, Cutner A, Cuckow P, et al. Laparoscopic gonadectomy in paediatric and adolescent girls with intersex disorders. BJOG 2003;110:210-2. [Crossref] [PubMed]
- Dénes FT, Cocuzza MA, Schneider-Monteiro ED, et al. The laparoscopic management of intersex patients: the preferred approach. BJU Int 2005;95:863-7. [Crossref] [PubMed]
- Moriya K, Morita K, Mitsui T, et al. Impact of laparoscopy for diagnosis and treatment in patients with disorders of sex development. J Pediatr Urol 2014;10:955-61. [Crossref] [PubMed]
- Kravarusic D, Seguier-Lipszyc E, Feigin E, et al. Androgen insensitivity syndrome: risk of malignancy and timing of surgery in a paediatric and adolescent population. Afr J Paediatr Surg 2011;8:194-8. [Crossref] [PubMed]
- Gad El-Moula M, Izaki H, El-Anany F, et al. Laparoscopy and intersex: report of 5 cases of male pseudohermaphroditism. J Med Invest 2008;55:147-50. [Crossref] [PubMed]
- Yu TJ, Shu K, Kung FT, et al. Use of laparoscopy in intersex patients. J Urol 1995;154:1193-6. [Crossref] [PubMed]
- Chertin B, Koulikov D, Alberton J, et al. The use of laparoscopy in intersex patients. Pediatr Surg Int 2006;22:405-8. [Crossref] [PubMed]
- Esposito C, Escolino M, Bagnara V, et al. Risk of Malignancy and Need for Surgery in Pediatric Patients with Morris or Y-chromosome Turner Syndrome: A Multicenter Survey. J Pediatr Adolesc Gynecol 2015;28:333-6. [Crossref] [PubMed]
- Burgmeier C, Leriche C. Laparoscopy in the Surgical Treatment of Disorders of Sexual Development. J Laparoendosc Adv Surg Tech A 2016;26:730-3. [Crossref] [PubMed]
- Wünsch L, Holterhus PM, Wessel L, et al. Patients with disorders of sex development (DSD) at risk of gonadal tumour development: management based on laparoscopic biopsy and molecular diagnosis. BJU Int 2012;110:E958-65. [Crossref] [PubMed]
- Looijenga LH, Hersmus R, de Leeuw BH, et al. Gonadal tumours and DSD. Best Pract Res Clin Endocrinol Metab. 2010;24:291-310. [Crossref] [PubMed]
- Ramirez PT, Frumovitz M, Wolf JK, et al. Laparoscopic port-site metastases in patients with gynecological malignancies. Int J Gynecol Cancer 2004;14:1070-7. [Crossref] [PubMed]
- Zivanovic O, Sonoda Y, Diaz JP, et al. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol Oncol 2008;111:431-7. [Crossref] [PubMed]
- Frydman R, Parneix I, Fries N, et al. Pregnancy in a 46, XY patient. Fertil Steril 1988;50:813-4. [Crossref] [PubMed]
- Nihoul-Fékété C. The Isabel Forshall Lecture. Surgical management of the intersex patient: an overview in 2003. J Pediatr Surg 2004;39:144-5. [Crossref] [PubMed]
- Oktay K, Bedoschi G. Oocyte cryopreservation for fertility preservation in postpubertal female children at risk for premature ovarian failure due to accelerated follicle loss in Turner syndrome or cancer treatments. J Pediatr Adolesc Gynecol 2014;27:342-6. [Crossref] [PubMed]
- Hewitt JK, Jayasinghe Y, Amor DJ, et al. Fertility in Turner syndrome. Clin Endocrinol (Oxf) 2013;79:606-14. [PubMed]


