Pediatric anesthesia for minimally invasive surgery in pediatric urology
Introduction
Use of laparoscopic and robotic surgery in pediatric population has been rapidly increasing over the last 10–15 years, and it’s become a standard of care for many of the operations involving the thoracic and abdominal cavities like some urological surgical procedure. Conventional and robotic pediatric laparoscopy offers many advantages over laparotomy, including decreased morbidity, rapid recovery, and improved aesthetics of incisions (1). However, minimally invasive surgery (MIS) does not means minimally-invasive anesthesia: though laparoscopic and robotic surgery don’t have same concerns about anesthesia as open abdominal surgery, usually they do introduce different ones, including physiologic effects of the pneumoperitoneum, absorption of CO2, and positioning required for surgery (it has to be noticed, in fact, that some laparoscopic procedures can take longer time than the open alternative). Moreover, although laparoscopy in children and adolescents seems to be similar to the one in adults, experience in adult surgery does not directly translate into safe surgery in younger patients. Pediatric procedures must be performed with a full understanding of the relevant anatomic and physiologic differences between the pediatric and adult populations.
Surgical considerations
Robotic surgery shows advantages over conventional laparoscopy (a better visualization of the surgical field, mechanical improvements, stabilization of instruments and improved ergonomics for the operating surgeon), so its use is increasing in common pediatric urologic surgeries (2). The application of minimally invasive technologies to urologic procedures in children includes both upper and lower urinary tract procedures as complete nephrectomy, partial nephrectomy, pyeloplasty, ureterocalicostomy, ureteroureterostomy, anti-reflux surgery, creation of continent catheterizable channels and augmentation cystoplasty (3,4). MIS often requires distension of the peritoneal cavity (except in a retroperitoneal approach), usually obtained by insufflation of CO2: it can be performed blindly using a Verres needle or placing of a port under direct vision through a small subumbilical incision. This latter approach is generally preferred in small children and infants because it can reduce the risk of abdominal viscera and vessel perforation (5). In adults, gas source is connected to the needle or port and intraabdominal pressure (IAP) is monitored while insufflating gas to aim for a pressure <15 mmHg to minimize pathophysiological effects. In infants and young children, insufflation pressures of 4 to 12 mmHg is typically suffice to visualize intraperitoneal structures and create operating space as far as the prepubertal abdominal wall is more pliable and the peritoneal cavity is smaller than in adults (6). The insertion of the laparoscope, after the expansion of the abdominal wall, grants to the surgeon the capability to easily observe intraabdominal space, to place instruments ports and to perform the procedure. A robotic system occupies a lot of space in the operating room and is made by a surgeon’s control console (remote from the patient), a stand for the optical system, and patient-side cart with robotic arms. After having created the pneumoperitoneum, then the surgeon can place ports for the camera and robotic arms, then controls them from the console. An assistant is at the patient’s side for suctioning, retraction, and passage of suture or sponges in and out of the abdomen.
Preoperative evaluation
Pediatric patients should always undergo appropriate preoperative evaluation for the planned procedure: a medical and anesthesia-directed physical examination have to be performed. A primary target in this stage is the evaluations of comorbidities that may impact the ability to tolerate surgery. In anticipation of laparoscopy, preoperative evaluation has to be focused on those medical conditions that may affect the response to physiologic changes associated with laparoscopy and surgical procedure, emphasizing even the particulars. Indeed congenital abnormalities of the genitourinary tract, often treated with MIS, may e.g., be associated with heart malformations (7) and can easily be used as a signal for the diagnosis of congenital heart disease. Insufflation of abdomen may pose an important risk in patients sensitive to decrease of ventricular preload. Management of these patients requires preoperative consultation with a pediatric cardiologist and intraoperative care by an anesthesiologist experienced in such conditions. Moreover in a congenital malformation scenario it’s important to seek abnormalities in central nervous system, respiratory tract and airways.
Physiopatologic effects of laparoscopy
Effects on cardiovascular system and regional circulatory changes
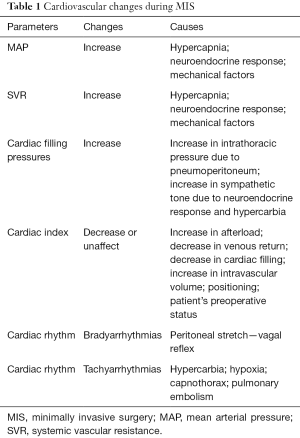
Hemodynamic events during laparoscopy, even if quite variable and deeply dynamic, are well known in the adult patient cases because of the more accurate studies done (Table 1). Authors have reported an increase in mean arterial pressure (MAP), systemic vascular resistance (SVR) and central venous pressure (CVP) with a decrease in cardiac output (CO) and stroke volume (SV): these effects are strictly related to the increase of IAP, to the effects of positioning and to absorption of CO2 (8,9). Pneumoperitoneum has both neurendocrine and mechanical effects on cardiovascular physiology. The increase in IAP may cause a catecholamine release and activation of the rennin-angiotensin system (10), increasing this way MAP and SVR. Mechanical effects depend on the patient’s preoperative volume status and IAP: compression of arterial and venous vasculature with pneumoperitoneum may increase SVR. Position has variable effects on CO and blood pressure: the head up position tends to reduce venous return to the heart and cardiac filling pressure; head down position, instead, increases venous return. CO2 has direct and indirect cardiovascular effects: it can directly increase SVR and the associated acidosis can decrease cardiac contractility, inducing sensitization to arrhythmias and causing systemic vasodilatation. Even if there is only a little information about the cardiocirculatory changes related to MIS in children, it seems to be similar to the ones in adults even at a lower IAP (11). However, in both infants and adults, these effects are generally well tolerated by healthy patients, thought vagal stimulation caused by insertion of the Veress needle or peritoneal stretch with gas insufflation, can result in bradyarrhythmias: it is crucial to consider that pediatric patients have a major risk of vagal reflexes and bradycardia during abdominal distension that may require emergent desufflation (12). Both mechanical and neurendocrine effects of pneumoperitoneum may decrease splanchnic circulation, while hypercapnia can cause splanchnic vasodilatation: changes on splanchnic circulation seems to be minimal and with no clinical impact (13). Cerebral blood flow and intracranial pressure could be increased (14) while renal blood flow reduced by the increasing of intraabdominal and intrathoracic pressure, hypercarbia and positioning (15): these alterations in a healthy patient are well compensated. Anyway the laparoscopic approach in patients with reduced intracranial compliance has to be generally avoided.
Full table
Effects on pulmonary system
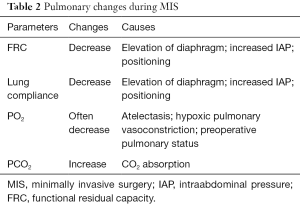
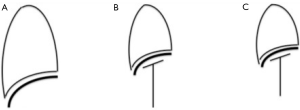
Pneumoperitoneum, positioning and absorption of CO2 could induce changes in pulmonary function and gas exchange (Table 2). Pneumoperitoneum causes cephalad displacement of the diaphragm, which reduces lung volumes and functional residual capacity (FRC), resulting in atelectasis, increased airway pressure for any given tidal volume and reduction in lymphatic drainage (16); these effects are exacerbated with Trendelemburg (Figure 1). The reduction in FRC and the atelectasis may lead to a ventilation/perfusion mismatch with intrapulmonary shunting and hypoxemia, caused by alveolar collapse (17). High rapidity in absorption, following the very high solubility of CO2, makes it necessary to increase ventilator rate: this is much truer in children, whose peritoneum is capable to absorb more gases than an adult one because of the shorter distance between capillaries and peritoneum, and the greater absorptive area in relation to body-weight. This can make it necessary to increase minute ventilation by over the 60% to restore end-tidal CO2 to baseline levels (18).
Full table
Anesthetic management
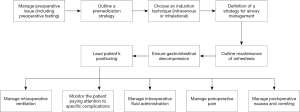
There is no dedicated anesthetic strategy to MIS in pediatric patients: the induction and maintenance are the standard pediatric anesthesia’s one. A flow chart summarizing the anesthetic management is provided (Figure 2). Recommendations for fasting in the pediatric population are the same used for adults, with the addition of guidelines for intaking of breast milk and infant formula. Children should be permitted to intake breast milk not later than 4 hours prior to surgery, and infant formula 6 hours prior (19). According to standard American Society of Anesthesiologists (ASA), monitors of blood pressure, electrocardiography, oxygen saturation, capnography, and temperature have to be applied before laparoscopy. Further monitoring (e.g., continuous intra-arterial pressure) could be based upon patient’s medical condition, expected blood loss, and duration of surgery. At least one venous catheter should always be placed; an additional venous access can be useful in case a blood loss is expected. For most children anxiolysis could be obtained thanks to distraction techniques during the induction of anesthesia rather than premedication with anxiolytics. The goal is to calm the child while avoiding the sedative and respiratory depressant effects of anxiolytics (20). When a pharmacological anxiolysis is required, midazolam is the drug of choice: it can be administered orally (0.3–0.5 mg/kg), rectally (0.5 mg/kg), sublingually (0.3 mg/kg) and nasally (0.3 mg/kg) (21). Atropine or glycopyrrolate may be included in premedication in order to prevent the reflex bradycardia induced by abdominal insufflations and to dry secretions blunting airway reactivity. Induction may be intravenous or inhalational: the choice largely depends on the ability of the child to tolerate placement of a IV catheter. In practice, this usually means that it should be preferred an inhalational induction for children in pre-scholar age, and an IV induction for older ones. However the decision has always to be individualized basing upon the child’s anxiety level. When compared with inhalational agents, IV induction agents are able to achieve more rapidly a level of anesthesia deep enough for airway instrumentation. Among these, propofol is the agent of choice as it provides rapid onset and short duration of action, reduces the bronchospastic response to intubation, and has antiemetic effects. When inhalational induction is chosen, most potent inhalational agents have the advantage of decreasing airway responsiveness: sevoflurane is generally preferred for induction because it is the least pungent comparing to isoflurane and desflurane. Desflurane is not generally used for inhalational induction because it is an extremely pungent volatile anesthetic that can produce an increase in secretions, coughing, airway resistance, and laryngospasm (22). After induction, an orogastric tube should be placed to decompress the stomach and the gut allowing minimizing stomach injury and increasing intraabdominal visibility. In relation to airway management for pediatric laparoscopy, endotracheal intubation is often preferred rather than a supraglottic airway (SGA): it provides optimal control of ventilation for elimination of CO2 and protection against aspiration (23). Standard practice in pediatric anesthesia includes the use of an uncuffed endotracheal tube (ETT) if the child is younger than 8 years old: this can make it difficult to maintain minute ventilation during the laparoscopy. The use of a cuffed ETT instead can allow the use of positive end expiratory pressure (PEEP) and high peak pressure along the airways during pneumoperitoneum. This far, ETT intubation with minimum cuff inflation can limit difficulties with ventilation (24). The use of SGAs for laparoscopy is controversial (25), and it has been used safely for short procedures (26). Maintenance of general anaesthesia during laparoscopy may be based on inhalational or intravenous agents, as it’s usually done for open abdominal procedures. The anesthetic is supplemented with intravenous opioids (e.g., Fentanyl or remifentanil) if needed. The use of nitrous oxide (N2O) is controversial: concerns regard an increase in postoperative nausea and vomiting and bowel distention (27). N2O diffuses into air containing closed spaces over time and can lead to bowel distention, being able to increase the technical difficulty of surgical maneuvers. Moreover it could have deleterious metabolic and neurotoxic effects in pediatric populations (28). Neuromuscular blocking agents are often administered during surgery to facilitate endotracheal intubation and to improve surgical conditions, but the literature about optimal level of neuromuscular blockade during laparoscopic surgery is inconsistent and the need of neuromuscular blockade may depend on the surgical procedure (29). Most of case requires controlled ventilation: as far as modern ventilators make it possible to have small tidal volumes delivered smoothly, a lung protective and volume targeted ventilation can be assured to pediatric patients. In fact, even if pediatric surgical data about ventilation outcome are missing now, lung-protective ventilation cannot be less than beneficial for sure, as it’s been observed in the adults. The strategy uses a target tidal volume in a range of 6–7 mL/kg, predicting use of PEEP to prevent atelectasis and, in case, recruitment maneuvers to revert it (16). Implementing such a strategy safely and effectively requires selecting the ventilation mode and monitoring the interaction between the ventilator and the patient to optimize the ventilator settings (30). Notwithstanding theory is clear, applying it tends to be challenging because of the difficulty to have exact bodyweight and optimal PEEP level in a pediatric patient: in this case, bedside monitors are a priority need, in order to let the operator choosing the optimal ventilation strategy, adjusting with a real time approach gas exchange, and thus ventilator parameters. Goals to achieve in this case are:
- Optimal arterial oxygen tension at least inspired oxygen concentration;
- Acceptable arterial CO2 tension;
- Delivered tidal volumes at least inspiratory pressure.
Another factor to be looked at, because of its postoperative outcomes, is the fluid management. Perioperative fluid requirements, in fact, depend upon multiple factors such as preoperative volume status, perioperative conditions, patient’s age, anesthetic management and nature of the interventions (laparoscopic procedures are associated with less insensible fluid losses than open ones). First goal in fluid therapy is to maintain euvolemia: it’s done by applying fixed volume algorithms to administer substantial amount of fluids even if, as it’s being observed, it can be easily obtained a decreasing of perioperative morbidity and then mortality by restricting intraoperative fluid administration. This is true both in adult and pediatric patients (31,32). Laparoscopy has been identified as a risk factor for PONV, therefore routine prophylactic multimodal antiemetic therapy should be utilized in all patients undergoing laparoscopic\robotic surgery (33). Postoperative pain after laparoscopic and robotic surgery is usually less than the corresponding open procedure, but the degree of pain depends on the specific surgery has been performed. Pain after laparoscopy can often be managed effectively with acetaminophen, nonsteroidal anti-inflammatory drugs, dexamethasone or opioids. Moreover, it could be useful infiltrate the incision with local anesthetic at the time of wound closure (34).
Complications
Both adult and pediatric procedures share similar complications, including those related to the physiologic effects of the laparoscopic approach (e.g., hemodynamic and respiratory decompensation, gas embolism), surgical maneuvers (vascular or solid organ injuries) and patient positioning (35). It’s necessary for the anesthesiologist to be aware of potential problems and being ready for a quick approach to them. The management of the complications (hypotension, hypertension and arrhythmias) includes confirmation that IAP is within acceptable limits, when all of treatable causes are excluded or resolved by the right supportive therapies (e.g., Reduction in anesthetics, fluid administration, pharmacologic interventions). It may even be necessary to deflate the abdomen if therapies are not effective and, eventually, to migrate to an open procedure. Hypercarbia or hypoxia could be instead respiratory decompensation signs related to the physiologic effects of the technique or to a surgical injury (e.g., Diaphragm injury). Hypercapnia is handled with the increase of ventilation aiming to compensate for CO2 absorption. Hypoxia can occur as a result of reduction in FRC and atelectasis caused by pneumoperitoneum and surgical positioning, or because of different reasons occurred during any anesthetic procedure. First chest should be auscultated to rule out a selective intubation or a bronchospasm, then the initial treatment should include an increasing of inspired oxygen concentration and, unless the patient is hypotensive, it should be performed both recruitment maneuver and PEEP optimizing. If refractory hypoxemia occurs, pneumoperitoneum must be desufflated; CO2 insufflation may also determine subcutaneous emphysema, capnothorax, capnomediastinum, capnopericardium, and gas embolism. Subcutaneous emphysema, in most cases, tends to resolves without specific interventions just after the abdomen has been deflated. CO2 absorption, in case of in significant subcutaneous emphysema, may continue for several hours after surgery, but healthy patients are able to increase ventilation to eliminated CO2: only patients with respiratory or cardiovascular problems should be observed in the post-anesthesia care unit until it resolves (36). Capnothorax, capnomediastinum and capnopericardium, although rare, may be life-threatening because they can be associated with severe hemodynamic compromise. They should be a matter of suspect in case of an unexplained increase of airway pressure, hypoxemia and hypercapnia with subcutaneous emphysema of head and neck or inequality of chest expansion. Reduction of insufflation pressure, increase in PEEP and hyperventilation are often sufficient to manage these syndrome, and only it’s rarely necessary to place an intrathoracic needle or a chest tube for decompression (37,38). Venous gas embolism is common during laparoscopy, but it is almost always subclinical: signs of suspect gas embolism include unexplained hypotension, hypoxemia and abrupt reduction of ETCO2. In case of suspect embolism the abdomen should be deflated, ventilation and fraction inspired oxygen increased to reduce dimensions of CO2 bubbles, and then supportive therapies with fluids and vasopressors administration may be helpful. Vascular, bowel, or bladder injury appears to be serious complications too: a survey about major complications of pediatric urological laparoscopy reported a rate of 1.2% (39). These occur mostly during initial entry or subsequent placement of trocars into the abdomen, as it usually happens in adult laparoscopy. Bleeding may be less obvious during laparoscopy than during open procedures. The view of the surgical field, in fact, is limited, and blood can pool away from the surgical field when patients are in head-up or head-down position. Signs of hypovolemia (e.g., hypotension, tachycardia) should be suggesting occult bleeding, and needs to be brought to the surgeon’s attention. Positioning is generally similar for pediatric and adults’ populations: care should be mostly taken to cushion pressure points on the arms, wrist and hand in order to avoid inadvertent nerve injury during the procedure. Usually most of urological surgeries are performed in the supine or lateral decubitus position, and this makes a steep Trendelemburg position not required as in adults’ procedures.
Conclusions
Nowadays conventional and robotic laparoscopic approach in pediatric surgery is widely used, even in younger patients including neonates. Because of its success in adults, these minimally invasive procedures have become a standard for the surgical treatment of many pediatric diseases, proving on the field to be safe and effective. However, it should be taken into account that, though the advantages (less postoperative pain, smaller incision with improved cosmesis, earlier oral intake, quicker mobilization and a shorter recovery), they can introduce particular pathophysiological implications for the anesthesiologist conduct.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mattei P. Minimally invasive surgery in the diagnosis and treatment of abdominal pain in children. Curr Opin Pediatr 2007;19:338-43. [Crossref] [PubMed]
- Herron DM, Marohn M, SAGES-MIRA Robotic Surgery Consensus Group. A consensus document on robotic surgery. Surg Endosc 2008;22:313-25; discussion 311-2. [Crossref] [PubMed]
- Traxel EJ, Minevich EA, Noh PH. A review: the application of minimally invasive surgery to pediatric urology: lower urinary tract reconstructive procedures. Urology 2010;76:115-20. [Crossref] [PubMed]
- Traxel EJ, Minevich EA, Noh PH. A review: the application of minimally invasive surgery to pediatric urology: upper urinary tract procedures. Urology 2010;76:122-33. [Crossref] [PubMed]
- Ahmed A. Laparoscopic surgery in children--anaesthetic considerations. J Pak Med Assoc 2006;56:75-9. [PubMed]
- De Waal EE, Kalkman CJ. Haemodynamic changes during low-pressure carbon dioxide pneumoperitoneum in young children. Paediatr Anaesth 2003;13:18-25. [Crossref] [PubMed]
- Nogueira PC, Paz Ide P. Signs and symptoms of developmental abnormalities of the genitourinary tract. J Pediatr (Rio J) 2016;92:S57-63. [Crossref] [PubMed]
- Hatipoglu S, Akbulut S, Hatipoglu F, et al. Effect of laparoscopic abdominal surgery on splanchnic circulation: historical developments. World J Gastroenterol 2014;20:18165-76. [Crossref] [PubMed]
- Meininger D, Westphal K, Bremerich DH, et al. Effects of posture and prolonged pneumoperitoneum on hemodynamic parameters during laparoscopy. World J Surg 2008;32:1400-5. [Crossref] [PubMed]
- Myre K, Rostrup M, Buanes T, et al. Plasma catecholamines and haemodynamic changes during pneumoperitoneum. Acta Anaesthesiol Scand 1998;42:343-7. [Crossref] [PubMed]
- Gueugniaud PY, Abisseror M, Moussa M, et al. The hemodynamic effects of pneumoperitoneum during laparoscopic surgery in healthy infants: assessment by continuous esophageal aortic blood flow echo-Doppler. Anesth Analg 1998;86:290-3. [PubMed]
- Terrier G. Anaesthesia for laparoscopic procedures in infants and children: indications, intra- and post-operative management, prevention and treatment of complications. Curr Opin Anaesthesiol 1999;12:311-4. [Crossref] [PubMed]
- Rist M, Hemmerling TM, Rauh R, et al. Influence of pneumoperitoneum and patient positioning on preload and splanchnic blood volume in laparoscopic surgery of the lower abdomen. J Clin Anesth 2001;13:244-9. [Crossref] [PubMed]
- Schöb OM, Allen DC, Benzel E, et al. A comparison of the pathophysiologic effects of carbon dioxide, nitrous oxide, and helium pneumoperitoneum on intracranial pressure. Am J Surg 1996;172:248-53. [Crossref] [PubMed]
- Chiu AW, Chang LS, Birkett DH, et al. The impact of pneumoperitoneum, pneumoretroperitoneum, and gasless laparoscopy on the systemic and renal hemodynamics. J Am Coll Surg 1995;181:397-406. [PubMed]
- Pelosi P, Vargas M. Mechanical ventilation and intra-abdominal hypertension: 'Beyond Good and Evil'. Crit Care 2012;16:187. [Crossref] [PubMed]
- Kalmar AF, Foubert L, Hendrickx JF, et al. Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth 2010;104:433-9. [Crossref] [PubMed]
- Pennant JH. Anesthesia for laparoscopy in the pediatric patient. Anesthesiol Clin North America 2001;19:69-88. [Crossref] [PubMed]
- Brady M, Kinn S, Ness V, et al. Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst Rev 2009.CD005285. [PubMed]
- Rosenbaum A, Kain ZN, Larsson P, et al. The place of premedication in pediatric practice. Paediatr Anaesth 2009;19:817-28. [Crossref] [PubMed]
- Kogan A, Katz J, Efrat R, et al. Premedication with midazolam in young children: a comparison of four routes of administration. Paediatr Anaesth 2002;12:685-9. [Crossref] [PubMed]
- Nyktari VG, Papaioannou AA, Prinianakis G, et al. Effect of the physical properties of isoflurane, sevoflurane, and desflurane on pulmonary resistance in a laboratory lung model. Anesthesiology 2006;104:1202-7. [Crossref] [PubMed]
- Patel A, Clark SR, Schiffmiller M, et al. A survey of practice patterns in the use of laryngeal mask by pediatric anesthesiologists. Paediatr Anaesth 2015;25:1127-31. [Crossref] [PubMed]
- Tobias JD. Anaesthesia for minimally invasive surgery in children. Best Pract Res Clin Anaesthesiol 2002;16:115-30. [Crossref] [PubMed]
- Saraswat N, Kumar A, Mishra A, et al. The comparison of Proseal laryngeal mask airway and endotracheal tube in patients undergoing laparoscopic surgeries under general anaesthesia. Indian J Anaesth 2011;55:129-34. [Crossref] [PubMed]
- Tobias JD, Holcomb GW 3rd, Rasmussen GE, et al. General anesthesia using the laryngeal mask airway during brief, laparoscopic inspection of the peritoneum in children. J Laparoendosc Surg 1996;6:175-80. [Crossref] [PubMed]
- Fernández-Guisasola J, Gómez-Arnau JI, Cabrera Y, et al. Association between nitrous oxide and the incidence of postoperative nausea and vomiting in adults: a systematic review and meta-analysis. Anaesthesia 2010;65:379-87. [Crossref] [PubMed]
- Baum VC. When nitrous oxide is no laughing matter: nitrous oxide and pediatric anesthesia. Paediatr Anaesth 2007;17:824-30. [Crossref] [PubMed]
- Kopman AF, Naguib M. Laparoscopic surgery and muscle relaxants: is deep block helpful? Anesth Analg 2015;120:51-8. [Crossref] [PubMed]
- Feldman JM. Optimal ventilation of the anesthetized pediatric patient. Anesth Analg 2015;120:165-75. [Crossref] [PubMed]
- Bundgaard-Nielsen M, Secher NH, Kehlet H. 'Liberal' vs. 'restrictive' perioperative fluid therapy--a critical assessment of the evidence. Acta Anaesthesiol Scand 2009;53:843-51. [Crossref] [PubMed]
- Mandee S, Butmangkun W, Aroonpruksakul N, et al. Effects of a restrictive fluid regimen in pediatric patients undergoing major abdominal surgery. Paediatr Anaesth 2015;25:530-7. [Crossref] [PubMed]
- Maitra S, Som A, Baidya DK, et al. Comparison of Ondansetron and Dexamethasone for Prophylaxis of Postoperative Nausea and Vomiting in Patients Undergoing Laparoscopic Surgeries: A Meta-Analysis of Randomized Controlled Trials. Anesthesiol Res Pract 2016;2016:7089454.
- Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol 2014;28:191-201. [Crossref] [PubMed]
- Gerges FJ, Kanazi GE, Jabbour-Khoury SI. Anesthesia for laparoscopy: a review. J Clin Anesth 2006;18:67-78. [Crossref] [PubMed]
- Kent RB 3rd. Subcutaneous emphysema and hypercarbia following laparoscopic cholecystectomy. Arch Surg 1991;126:1154-6. [Crossref] [PubMed]
- Joris JL, Chiche JD, Lamy ML. Pneumothorax during laparoscopic fundoplication: diagnosis and treatment with positive end-expiratory pressure. Anesth Analg 1995;81:993-1000. [PubMed]
- Venkatesh R, Kibel AS, Lee D, et al. Rapid resolution of carbon dioxide pneumothorax (capno-thorax) resulting from diaphragmatic injury during laparoscopic nephrectomy. J Urol 2002;167:1387-8. [Crossref] [PubMed]
- Peters CA. Complications in pediatric urological laparoscopy: results of a survey. J Urol 1996;155:1070-3. [Crossref] [PubMed]