Reverse Szabo technique for stenting a single major aorto-pulmonary collateral vessel in pulmonary atresia with ventricular septal defect
Introduction
Pulmonary atresia with ventricular septal defect (PA-VSD) is a complex heart lesion, occurring 2% of all congenital malformations (1,2). Management of patients with PA-VSD in the neonatal period presents numerous challenges (3). The primary aim of intervention for these patients is to provide reliable pulmonary blood flow in order to prevent life-threatening desaturation and promote further growth of the pulmonary arteries. The traditional approach includes a prostaglandin E infusion started shortly after birth with subsequent surgical systemic-to-pulmonary artery shunting. However, in 25% of cases with this lesion, the source of pulmonary blood supply are collateral vessels, either single or multiple, instead of the ductus arteriosus. These collateral vessels are typically not amenable to the effects of prostaglandin, and tend to be stenotic, making conservative management of such patients fairly unpredictable.
In addition, application of an aorto-pulmonary shunt, particularly in the setting of right aortic arch, which occurs in 40% of patients with PA-VSD, is technically more challenging, often requires a sternotomy with cardiopulmonary bypass, and could create significant PA distortion (1,4).
Recently, endovascular stenting of the ductus arteriosus or of a collateral vessel in ductal-dependent pulmonary circulation as an alternative to the BT-shunt has become increasingly popular (5-7). In PA-VSD, the pulmonary trunk is usually absent or severely hypoplastic. This results in changing the pulmonary artery bifurcation geometry, which makes positioning of the distal end of the stent particularly difficult without the risk to compromise blood flow in to the PA branches.
We describe the reverse Szabo (anchor-wire) technique, which is used for precise proximal stent positioning at the main branch in bifurcation coronary stenting (8,9). In the available literature, we found only a very limited description of the similar technique in congenital heart defects, by Girona et al., describing a similar concept but with a slightly different modification (10).
Case presentation
Patient M., 2 days of age, 2.5 kg, with a prenatal diagnosis of “Tetralogy of Fallot” and without any significant comorbidities, was referred from the neonatal hospital in clinically stable condition with saturation of 90%. Transthoracic echocardiography revealed pulmonary atresia with ventricular septal defect, and a continuous infusion 20 ng/kg/min of PGE1 was started. During the following 10 days the patient’s condition remained stable, but the saturations gradually decreased to 70% without significant response on to increased prostaglandins.
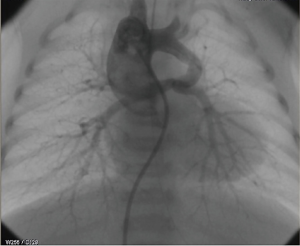
On the 12th day of life, the baby was taken for catheterization, which confirmed PA-VSD, single major aorto-pulmonary collateral artery (MAPCA) and, right-sided aortic arch (Figure 1). No other significant sources of pulmonary flow were found.
Venous access was obtained via the right common femoral vein with a 4 Fr introducer sheath (Terumo, Tokyo, Japan) and IV Heparin 100 IU/kg was administered. A 4-Fr JR catheter over 0.035'' hydrophilic angled guide wire (Radifocus, Terumo, Tokyo, Japan) was advanced through the VSD into the ascending aorta. A 0.018'' guide wire (V-18, Boston Scientific, Marlborough, MA, USA) was then inserted into the descending aorta, after which the 4-Fr introducer sheath was exchanged for a long 4-Fr sheath (Cook Medical, Bloomington, IN, USA) positioning the tip in the ascending aorta opposite the innominate artery. The innominate artery was engaged by rotating of the sheath, and the 0.018'' guide wire was advanced through the collateral vessel into the distal pulmonary artery followed by the sheath, deeper to the ostium of the collateral vessel. To facilitate further insertion of the sheath further and establish a more stable position, we used the “mother and child” technique by introducing the 4-Fr JR catheter through the sheath deeper into the collateral vessel. Then the pulmonary arteries were wired with two 0.014’’ guide wires (Runthrough NS, Terumo, Tokyo, Japan) as distally as possible, in order to achieve a stable position of the assembly (Figure 2). After measuring collateral vessel length and considering patient weight, the coronary bare metal stent 3.5×22 mm2 was selected (Integrity, Medtronic, Santa Rosa, CA, USA).
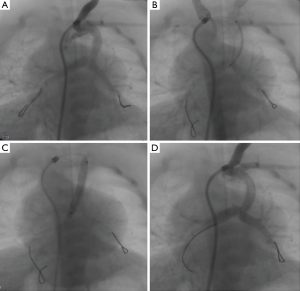
The proximal end of the right pulmonary artery guide wire was introduced into the lumen of the balloon. Then the protective tube over the stent was slightly withdrawn to uncover two rows of stent cells. The balloon was gently inflated to expand the most distal rows of stent cells (Figure 3A). The second left pulmonary artery guide wire (anchor wire) was inserted through the most distal, expanded stent cell (Figure 3B) and the stent was manually crimped over the balloon again (Figure 3C). The stent was then advanced to the branch pulmonary artery bifurcation (Figure 2B) and, after confirming its location with angiography, deployed at nominal pressure (Figure 2C).
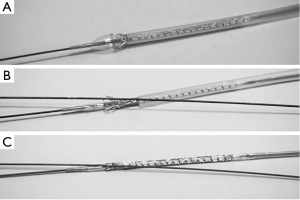
With this technique, the anchor wire delivers the distal position of the stent at the bifurcation precisely and prevents undesired distal sliding of the stent during inflation (Figure 2D). At the same time, we can safely apply continuous forward force on the whole assembly to prevent the stent from missing the bifurcation, which typically is the most stenotic segment.
The post-intervention course was without complications. After the procedure, the patient developed moderate pulmonary over-circulation with saturations of 96%, which were easily controlled with conservative medical management. The patient was extubated the following morning, his condition was stable with saturations of 88–90% without heart failure or systemic hypoperfusion. Two days after the procedure, he was transferred to the neonatal hospital on Aspirin 5 mg/kg per day.
Discussion
The anchor-wire technique, originally described by Szabo, was used in coronary stenting for precise positioning of the stent in the ostium of the main vessel (8). The Reverse Szabo technique at V-stenting of the coronary arteries was described by Lo and Kern (9). Nonetheless, the use of anchor-wire technique for stenting in congenital heart diseases has been limited. Only Girone et al. described the use of an extended anchor-wire concept in the treatment of congenital malformations (10). However, in their original description, to anchor the distal end of the stent, the anchor wire was passed through the entire stent. In our modification, the anchoring wire was passed through the most distal stent cell. As this is a pre-mounted assembly, we were able to minimize the risk of the stent sliding from the balloon while advancing.
Conclusions
The utilization of the described technique for PDA/collateral vessel stenting has the following advantages over the traditional approach: (I) precise positioning of the distal end of the stent exactly at the bifurcation without the risk of stent protrusion into the one of the branch pulmonary arteries; (II) minimization of the risk of missing the usual stenosis at the insertion of the vessel at the pulmonary artery bifurcation by allowing safe continuous forward pressure application on the stent-balloon assembly before and during deployment; (III) and facilitate advancement of the stent-balloon assembly due to the presence of a buddy-wire.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this mauscript and any accompanying images.
References
- O'Leary PW, William D, Edwards WD, et al. Pulmonary Atresia and Ventricular Septal Defect. In: Moss and Adams' Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult. Vol. 2. Chapter 42. Philadelphia: Wolters Kluwer Health / Lippincott Williams & Wilkins, 2008.
- Haas NA, Kleideiter U. Pulmonary Atresia with Ventrical Septal Defect. In: Haas NA, Kleideiter U. Pediatric cardiology: symptoms, diagnosis, treatment. Stuttgart: Thieme 2015:51-4.
- Lofland GK. The management of pulmonary atresia, ventricular septal defect, and multiple aorta pulmonary collateral arteries by definitive single stage repair in early infancy. Eur J Cardiothorac Surg 2000;18:480-6. [Crossref] [PubMed]
- Muralidhar K. Modified Blalock Taussig shunt: comparison between neonates, infants and older children. Ann Card Anaesth 2014;17:197-9. [PubMed]
- Alwi M. Stenting the ductus arteriosus: Case selection, technique and possible complications. Ann Pediatr Cardiol 2008;1:38-45. [Crossref] [PubMed]
- Alwi M, Choo KK, Latiff HA, et al. Initial results and medium-term follow-up of stent implantation of patent ductus arteriosus in duct-dependent pulmonary circulation. J Am Coll Cardiol 2004;44:438-45. [Crossref] [PubMed]
- Okubo M, Benson LN. Intravascular and intracardiac stents used in congenital heart disease. Curr Opin Cardiol 2001;16:84-91. [Crossref] [PubMed]
- Szabo S, Abramowits B, Vaitkuts PT. New technique for aorto-ostial stent placement (Abstr) Am J Cardiol 2005;96:212. H.
- Lo H, Kern MJ. Use of a branch wire to anchor stents for exact placement proximal to bifurcation stents: the reverse Szabo technique. Catheter Cardiovasc Interv 2006;67:904-7. [Crossref] [PubMed]
- Girona J, Martí G, Betrian P, et al. Extended Szabo (anchor-wire) technique concept for stent implantation in congenital heart lesions. Pediatr Cardiol 2012;33:1089-96. [Crossref] [PubMed]


