Prophylactic arrhythmia surgery in association with congenital heart disease
Introduction
The idea that prophylactic arrhythmia surgery should be incorporated into reparative open heart procedures stems from the reality that many patients with specific congenital cardiac anatomic substrates are subject to atrial arrhythmia development in the course of their lives, which will impact negatively on ventricular function, physical well being, and long-term survival (1-3). Patients presenting later in life with any form of atrial septal defect (ASD) have a 30% to 50% incidence of atrial arrhythmias [mostly atrial fibrillation (AF)] with or without operative repair (4-8). Patients with Ebstein anomaly of the tricuspid valve and patients undergoing repeat surgery for tetralogy of Fallot (TOF) are at significantly increased risk of atrial arrhythmia development (9-11). Patients who have had staged procedures en route to Fontan physiology also have a high incidence of atrial arrhythmias whether the connections be atriopulmonary, total cavopulmonary, or extracardiac/lateral tunnel (12-15). Others with complex atrial baffles such as atrial switch procedures in association with arterial switch (double switch for congenitally corrected transposition of the great arteries) are associated with a predictable incidence of atrial arrhythmias, which theoretically can be ameliorated or neutralized (mitigated) by a prophylactic maze procedure.
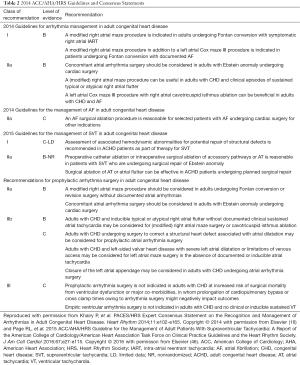
Presently, there is emerging consensus regarding indications for prophylactic arrhythmia surgery in congenital heart disease (16). The surgical community has not reached unanimity of opinion as to what constitutes a standard prophylactic maze procedure (1,17-21). While the operation was conceived to be complication-free owing to the lesions being placed in areas that theoretically do not interfere with normal sinus rhythm mechanism and one-to-one conduction, the reality is that there have been reported cases of sinus node dysfunction resulting in nodal rhythm following maze procedures (21,22). Understandably, one must approach such a conundrum with a rationale as to which forms of heart disease are associated with sufficiently high risk of developing arrhythmia (Table 1) (16) to warrant consideration for prophylactic arrhythmia surgery, as well as clarity regarding the set of prophylactic lesions to be performed, the appropriate lesion sets, and techniques. These considerations are important in light of the fact that prophylactic arrhythmia therapy may be performed without advanced knowledge that the patient in question will actually develop an arrhythmia during the course of his or her life. Invocation of bioethical principles of non-maleficence, beneficence, patient autonomy, and justice all come to mind and apply (1,23-25). The idea is to establish the historic incidence of these arrhythmias, identify the arrhythmia (right-sided, left-sided, both right and left sided) and offer a safe, effective, and complication-free prophylactic procedure (4,22,26-28).

Full table
The aim of this review is to identify preoperative congenital heart surgery patients who are at risk for developing future atrial arrhythmias, assess the efficacy of techniques of arrhythmia surgery in treating the specific arrhythmia substrate, and assess the risk/benefit of prophylactic arrhythmia surgery. Concomitant prophylactic arrhythmia surgery in association with reparative procedures is discussed based on a literature review and considered application of safe lesion sets for standardization and future interprogram comparisons.
Arrhythmias and congenital heart disease
The natural history of unrepaired and repaired congenital heart disease is fraught with late arrhythmogenic complications and therefore is an important field of inquiry. As the complexity of types of congenital heart disease undergoing surgery advanced, the recognition of associated arrhythmia development as a significant source of late morbidity evolved. The marked improvement in survival among patients with congenital heart disease has been associated with the recognition that late arrhythmias and heart failure account for over half of late deaths (29,30). Among adults with congenital heart disease, the development of atrial arrhythmias is associated with a 50% increase in early mortality, a two-fold increase in stroke and congestive heart failure, and a three-fold increase in the need for cardiac interventions (31). Lesions associated with the highest prevalence of supraventricular tachycardia (SVT) include Ebstein anomaly, atrial repairs of transposition of the great arteries, univentricular hearts, ASDs, and right heart obstructive lesions such as TOF and double outlet right ventricle (31). Of these defects, more than 25% of patients with right heart obstructive lesions, Ebstein anomaly, and univentricular hearts undergo reoperations (10,32). Patients with ASDs may present for intervention in adulthood (1,6,8). Clearly, a surgical intervention that reduces the risk of later arrhythmia development by the inclusion of successful prophylactic arrhythmia surgery should result in improved quality of life for a significant number of patients (1).
ASDs, although technically straightforward substrates for surgical repair, are associated with late atrial flutter (AFL) and AF in as many as 20% to 35% of patients (6,26,33). In 1990, Murphy et al. studied 123 patients with ostium secundum or sinus venosus ASD in an effort to determine the natural history of surgically corrected ASDs, 27 to 32 years following the repair (6). Patients repaired before age 25 had excellent prognosis; patients aged 25 to 41 had good survival but less than age matched controls; and patients greater than 41 years had poor survival and more frequent late cardiac failure, stroke, and AF. Their results indicate “that age at operation is the most powerful independent predictor of long-term survival”. The idea that ASD closure after 40 years of age is associated with increased risk of late complications and arrhythmias was heralded by this study (6). Gatzoulis and associates [1999] from Toronto retrospectively identified 213 adults who underwent surgical ASD closure owing to symptoms or substantial left-to-right shunt (ratio of pulmonary to systemic blood flow >1.5:1), or both (26). In comparison with patients who did not have preoperative AF or AFL, the 40 patients with AF or AFL were older and had higher pulmonary artery pressure, and 24 of the 40 patients (60%) continued with AF or AFL after mean follow-up of 3.8 years. New onset AF or AFL was found at follow up at greater frequency in patients who were older than 40 years at the time of surgery, echoing Murphy’s 1990 report (6). The authors concluded via multivariate analysis that older age at time of surgery (>40 years; P=0.001); presence of preoperative AF or AFL; and presence of postoperative AF, AFL, or junctional rhythm were predictive of late postoperative AF or AFL in adults with ASD (26). In Belgium, 155 patients who underwent ASD closure were selected from 3 databases (33). All patients were 18 years or older; 24 were surgically repaired, and 131 underwent transcatheter device closure (33). Over a median follow-up of 25 months (range, 1–289 months), 25% developed atrial arrhythmia. Risk factors for arrhythmia development were preoperative or early postoperative atrial tachycardia, female gender, and mean pulmonary artery pressure ≥25 Torr (33).
Arrhythmia development following surgical repair of TOF was initially focused on the risk of ventricular tachycardia, related to ventriculotomy, fibrosis, scarring and ventricular dilatation and hypertrophy. Subsequently, as surgical techniques evolved and repairs were performed at younger ages, atrial arrhythmias were recognized in over 40% of patients, which contributed to important morbidity and hospitalizations (11). Ebstein anomaly of the tricuspid valve is associated with SVT in 20% to 50% of patients, related to accessory connections as well as AF and AFL (9,19,34). Perhaps the most challenging patients are those with postoperative Fontan complications who develop atrial arrhythmias with increasing incidence over time which can be as high as 50% (32,35). Oftentimes, these patients present with gigantic right atria, atrial reentry tachycardia, AF, and hemodynamically important lesions requiring surgery such as: venous and arterial pathway obstructions, valvar insufficiency, aneurysms, and intracavitary clot formation. Modification of the Fontan operation has decreased the incidence of late atrial tachycardia to approximately 8% to 15% in extracardiac connections, 13% to 60% in lateral tunnel connections, and over 60% in the earlier atriopulmonary connection repairs (36). Incidence of late arrhythmias in patients with modified connections can be expected to rise with longer follow-up. Catheter ablation in the Fontan patient has acute success rates of about 50% with at least 70% recurrence of tachycardia within two years (37,38). Catheter access to the right atrium in patients with extracardiac connections is limited to the transhepatic or transthoracic approach with potential morbidity. Certainly patients with prior Fontan surgery undergoing reoperations should be considered for prophylactic lesion sets, taking on greater importance in light of limited transcatheter access.
Arrhythmia surgery
The historic record of arrhythmia surgery has been mostly confined to therapeutic application of specific lesion sets developed to treat existing refractory arrhythmias with or without associated intracardiac repair. Sealy and Cox originated the descriptions of surgical treatment of accessory connections and successfully applied the techniques to hundreds of patients (39). Guiradon et al. extended surgical therapy to patients with AFL by developing the isthmus lesion from the coronary sinus to the inferior vena cava (IVC) (40). Theodoro and colleagues applied the techniques of the right sided maze as described by Cox to patients with congenital heart disease and atrial arrhythmias (21). Mavroudis extended the application of surgical treatment of both AFL and AF to patients with congenital heart disease of all ages and to patients with prior Fontan surgeries (1,15,41-43). During preoperative electrophysiologic studies the recognition of three dominant reentrant circuits for atrial tachycardia in Fontan patients led to the development of the modified right atrial maze, which included an isthmus lesion that was not part of the Cox right atrial maze (12,15). Multiple centers have successfully applied these various operative arrhythmia techniques to patients with congenital heart lesions.
In 2006, Karamlou and colleagues have demonstrated efficacy of concomitant atrial arrhythmia surgery in TOF patients with preexisting atrial arrhythmias who were undergoing pulmonary valve surgery and or tricuspid valve repair (44). In patients without arrhythmia by 18 years of age, reported SVT prevalence during long-term follow-up was over 15% (3). Giamberti and colleagues published their cumulative experience of 50 adults with congenital heart disease in 2006 (8) and 2008 (45). Patients underwent irrigated radiofrequency ablation concomitantly (31 right-sided maze procedures; 13 Cox-maze III procedures; 6 right ventricular ablations; and additional 14 pacemakers). Two patients died from causes not related to intraoperative ablation. During average follow up of 28 months, 48 patients were alive and in New York Heart Association class I or II. All patients were discharged with antiarrhythmic medication for 3 months. Forty three patients were still in sinus rhythm, 2 were in sinus rhythm and taking permanent antiarrhythmic medication for recurrent AF, 2 were in stable AF, and 1 was in pacemaker rhythm at the time of publication (45). The authors found irrigated radiofrequency ablation to be effective to control arrhythmias in adults with congenital heart disease (45).
Recognizing the increasing contribution of arrhythmias to long-term morbidity, three recent groups have published recommendations for concomitant arrhythmia surgery in patients with existing arrhythmias undergoing planned surgical repairs (Table 2) (16,46). The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines for the management of SVT in adults include a class I recommendation for assessment of associated hemodynamic abnormalities for potential repair in adults with congenital heart disease as part of therapy for SVT (46). In the consensus statement for management of arrhythmias in adults with congenital heart disease, surgical ablation of associated atrial tachycardia is recommended in patients undergoing planned surgical repair, and a left atrial Cox-maze III procedure with right atrial cavotricuspid isthmus ablation is recommended for adults with congenital heart disease and AF. Guidelines for the management of AF in adults consider it reasonable to perform surgical ablation for AF in select patients undergoing cardiac surgery for other indications. These guidelines were made in recognition of the efficacy of surgical techniques for treating AFL and AF, as well as SVT related to accessory connections (16,46).
Full table
Arrhythmia surgery techniques
AF
The original maze procedures for AF were characterized as Cox-maze I, II, and III (47-49). Because original lesion sets were designed as “cut and sew”, energy ablative sources were introduced to shorten the procedure and limit bleeding complications, termed the Cox-maze IV. Lesion sets were designed to isolate left atrial macro and micro-reentry and prevent AF, while preserving conduction from the sinoatrial node to the atrioventricular node to maintain atrioventricular synchrony, preserve left atrial transport function, and reduce thromboembolism. The left atrial maze procedure is effective owing to the specific lesions designed to encircle the pulmonary veins and to limit reentry circuits that would occur in the left atrioventricular valve isthmus, reentry via the coronary sinus, and reentry via Bachmann’s bundle in the dome of the left atrium (1). Originally, the Cox-maze procedure included left atrial appendectomy and an incision to the confluence of pulmonary vein encircling lesion(s). Resection of the left atrial appendage was thought to remove the source of thrombi known to occur in AF; it is not clear whether the left atrial appendectomy plays a role in arrhythmia ablation.
Cox articulated the following observations regarding AFL/AF and the maze procedure (50):
- The local effective refractory periods of the left atrium are shorter than in the right atrium;
- AFL most likely occurs on the basis of reentry in the right atrium (longer effective refractory periods and larger reentrant circuits);
- AF likely occurs on the basis of reentry in the left atrium (shorter effective refractory periods and smaller reentrant circuits);
- Maze incisions confined to the left atrium are likely to ablate AF but not AFL (50).
Subsequent modifications to the maze procedure, all developed to shorten the operation, allow epicardial approaches without cardiopulmonary bypass and facilitate a transcatheter ablation (1,51,52). Further, because AF typically originates in the left atrium, potential exclusion of the right-sided lesions was introduced (50,51). Subsequent efforts to minimize the pulmonary vein and left atrial lesions resulted in procedures labeled as mini-maze, “modified” maze, “right atrial” maze, ”left atrial” maze, and “biatrial” maze. “Maze” became synonymous with any modified lesion set that was applied to the atria as therapy for reentrant atrial arrhythmias.
The original “cut and sew” Cox-maze III procedure resulted in long-term freedom from AF in greater than 97% of patients (1,49,53). All subsequent modifications have achieved varying degrees of efficacy, approaching 93% (1,54-56). The superiority of a biatrial maze procedure for AF prevention has been demonstrated in several studies (1,17,55,56). In a review of many different lesion sets for AF, Barnett and Ad [2006] reviewed 69 studies and 5,885 patients who underwent surgical ablation (67% biatrial and 33% left atrial) for AF lasting >6 or 12 months (56). Survival rates were similar for both procedures, however, the biatrial maze ablation demonstrated superior long-term freedom from AF at all time points.
AFL
The classic “cut and sew” right-sided maze procedure (49) involves a linear incision from the superior vena cava (SVC) to the IVC, right atrial appendectomy, incision from the base of the resected right atrial appendage to the midpoint of the right atrial anterior wall not in communication with the SVC-IVC incision, an incision posteriorly from the base of the right atrial appendage to the anterior tricuspid valve annulus, and a communicating incision from the SVC-IVC incision to the posterior tricuspid valve annulus (1). It is important to recognize that these lesions were developed from animal models without congenital heart disease or previous operations.
Subsequent electrophysiology studies have demonstrated the key role played by the right atrial isthmus in typical right AFL (isthmus dependent right atrial macroreentry) (1,57-60). The right atrial isthmus is considered the area between the tricuspid valve annulus and the coronary sinus and the IVC. Targeted ablation of this isthmus region transforms the area of “slow conduction” to an area of “no conduction” and effectively terminates typical AFL (1,61).
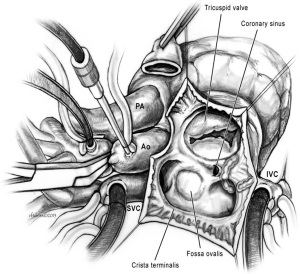
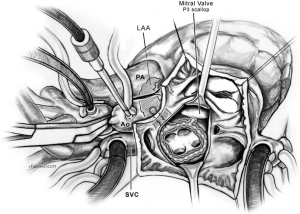
In the congenital heart disease population, additional right atrial macroreentrant circuits have been identified commonly referred to as “non-isthmus” dependent tachycardia (1,62). These circuits may involve reentry around prior incisions (“incisional tachycardia”) or prosthetic material such as ASD patches. The lateral right atrial wall at the inferior aspect of the crista terminalis is often an area of unexcitable atrial tissue with low voltage electrograms and is labeled as “scar”. This area of “slow conduction” or scar can contribute to an additional macroreentrant circuit. Elimination of the isthmus of slow conduction between these incisions, patches, or electrical scars forms the basis of ablation strategies for non-isthmus-dependent right atrial tachycardia. These alternative lesion sets are referred to as “modified right atrial maze procedures” and appear to be responsible for elimination of right atrial tachycardia in the setting of complex congenital heart disease (14,62). The lesion sets of the modified right atrial maze (Figures 1,2) (1,63) may not be appropriate to employ as prophylactic lesion sets as the evidence for first time arrhythmia occurrence favors an isthmus-dependent circuit. In light of the accumulated retrospective studies, we favor an isthmus lesion (Figure 1) for right atrial arrhythmia prophylaxis (1,63).
Energy sources
Technical concerns relative to the “cut and sew” maze include the length of the procedure and risk of perioperative bleeding (1). Energy sources have been developed to minimize the need for incisions and subsequent bleeding complications (1,8,48-51,53,64).
Khargi and colleagues [2005] compared alternative sources of energy (radiofrequency-microwave and cryoablation; group I) for treating AF with a classic cut and sew Cox-maze III (group II), which claims 97% to 99% sinus rhythm success rate (1,65). Forty eight studies were reviewed with 3,832 patients (2,279 in group I and 1,553 in group II). There was no difference in mean duration of preoperative AF, left atrial diameter and left ventricular ejection fraction. Freedom from AF was 78% (group I), 85% (group II), and not statistically significant, implying no significant difference in the two sources of energy (1). Schuessler et al. [2009] summarized their experience in porcine models with 9 different unidirectional devices to create continuous transmural lines of ablation from the atrial epicardium and thereby replace the cut and sew lesions with lines of ablation and perform the procedure without cardiopulmonary bypass (66). The devices included radiofrequency, microwave, lasers, and a cryothermia device. The maximum penetration of any device was 8.3 mm and therefore all devices except one (a radiofrequency device) failed to penetrate 2.0 mm in some non-transmural sections.
It appears that depth of lesions by whatever means is more important than the energy sources/incisions that are used to achieve the result. Patients with congenital heart disease have varying degrees of atrial thickness owing to the specific heart defect and the adaptive mechanisms over a lifetime of perturbed hemodynamics. For example, patients with tricuspid atresia have thick atrial walls, while patients with double inlet left ventricle tend to have thin atrial walls. These anatomic variances become important when a transmural lesion needs to be accomplished.
What we know about lesion sets for atrial tachycardia
The right-sided maze procedure as described by Cox et al. [1991] has a number of lesion sets that are performed by the classic “cut and sew” technique (47). The traditional surgical lesions in the classical right atrial maze include right atrial appendectomy, a lesion between the amputated right atrial appendage and the anterior tricuspid annulus, and the lesion from the SVC to the IVC (63). In particular, the lesion between the SVC and the IVC is similar to the incision that is oftentimes performed to repair a sinus venosus ASD (63). This incision is commenced in the upper third of the right atrial free wall and extends through the area of the sinoatrial node into the SVC. This repair, when performed in this manner has a high incidence of sinus node dysfunction, resulting in nodal rhythm.
Subsequent development of the modified right atrial maze includes lesions between the IVC and the tricuspid valve, the coronary sinus and the tricuspid valve, and between the IVC and the coronary sinus. The right atrial cavotricuspid isthmus represents areas of “slow conduction” which together with an area of unidirectional block contribute to atrial reentry tachycardia (1,61,63). Converting these locations from areas of slow conduction to areas of no conduction using transcatheter radiofrequency ablation techniques can be effective. This area at the so-called “isthmus” is usually the first set of applied radiofrequency lesions that are delivered by transcatheter techniques in a hierarchical series that includes the area between the fossa ovalis and the lateral wall crista terminalis, the base of the atrial appendage and the tricuspid annulus, as well as other areas of slow conduction that are identified by electrophysiologic mapping.
Because of the severity of the tachycardia in patients with complex congenital heart disease such as Fontan patients, arrhythmia ablation tends to be more important than the risk of nodal rhythm, which can be treated by pacemaker therapy (63). In addition, surgical ablation in these populations does not lend itself to a step wise hierarchical treatment plan because access to the anatomic areas of interest often require extending the cross clamp time under some type of systemic hypothermia. Separation from cardiopulmonary bypass, remapping and recommencement of cardiopulmonary bypass/aortic cross clamp to ablate the next area of interest increases the risks of myocardial ischemia, systemic inflammation, and air embolism (63). As a result, surgeons are more likely to ascribe to the axioms “more is better” and “getting it right the first time”. These tenets are not operative, however, when considering simple cases of atrial tachycardia or recent onset of AF in patients with two ventricles and congenital heart disease. This is especially true when considering prophylactic arrhythmia surgery, which raises the tenet of “do no harm”.
In light of evidence of sinus node dysfunction following the classic right atrial maze in the ASD population, it seems unwise to include the lesion between the SVC to IVC to prevent atrial tachycardia as a prophylactic procedure, especially because there is little proof as to the validity of this lesion when compared with the isthmus ablation line. The same is true for the lesion that is sometimes placed from the fossa ovalis to the posterior atrial flap of the atriotomy, when an atriotomy is required for right atrial access. While this lesion might be indicated for treatment of incessant atrial tachycardia, there is very little evidence that such a lesion might be effective as a prophylactic measure.
Proposed lesions sets for prophylactic arrhythmia surgery
Prophylactic arrhythmia surgery in humans with congenital heart disease has not been tested by a prospective, randomized, clinical study owing to non-standardized lesion sets, variable patient populations, and lack of unanimity for the need of this additive procedure, which requires a measurable amount of cardiopulmonary bypass and cross-clamp times (1). In addition to these impediments are the paucity of retrospective clinical studies, which can help to define various approaches to certain patient populations and can form the basis for clinical equipoise and the need for an organized multi-institutional study (1).
There have been very few studies that have applied therapeutic lesion sets as prophylactic measures in humans (1,5,17,21). Based on animal studies that induce atrial tachycardia and lysis with one incision, Collins and associates applied a single incision in the anterior atrial flap to the anterior tricuspid annulus in Fontan patients (67). The idea was that this lesion would mitigate against the incidence of atrial tachycardia. The short-term results failed to show efficacy, which was perhaps related to the small number of patients with limited follow up or alternatively related to a lesion set that did not address the right atrial cavotricuspid isthmus (1).
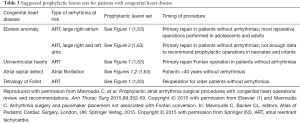
Based on the limited clinical studies, retrospective surgical and transcatheter ablation results, and the debated opinion of surgeons, prophylactic arrhythmia lesion sets are offered for diagnostic subsets with predictive arrhythmia occurrence that are undergoing a primary or secondary therapeutic anatomic surgical intervention (Table 3, Figures 1,2) (1,63). Based on the historic data regarding populations with the highest incidence of atrial arrhythmia development, targeted populations for prophylactic arrhythmia surgery in the right atrium include patients with unrepaired ASDs presenting over 40 years of age (1,5), patients with Ebstein anomaly (1), tetralogy patients presenting for pulmonary valve insertion (1,68,69), and single-ventricle patients who present for Fontan operations (1,16,32,70-72). Prophylactic surgery for AF would be considered for patients with significant left-sided atrioventricular disease and severe left atrial dilatation undergoing planned surgery, with lesions including left atrial maze and right-sided cavotricuspid isthmus ablation (16).
Full table
Figure 1 shows a lesion set that interrupts the potential areas of slow conduction at the “isthmus” (1,63). This is the first area that is approached for therapeutic transcatheter radiofrequency ablation in patients with atrial reentry tachycardia, which is successful in 75% of cases. The area of interest is easy to locate, easy to ablate and has minimal risks. Figure 2 shows the lesion set for prevention of AF.
Lesion sets should be standardized not only for therapeutic measures but also for prophylactic applications for patients with congenital heart disease undergoing repair. The principles of prophylactic procedures should be preserved, namely that the prophylactic procedure should be simple to perform, should be attended by a minimum of complications, and be supportive of potential problems that can cause significant hemodynamic problems to the patient over the long term.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mavroudis C, Stulak JM, Ad N, et al. Prophylactic atrial arrhythmia surgical procedures with congenital heart operations: review and recommendations. Ann Thorac Surg 2015;99:352-59. [Crossref] [PubMed]
- Koyak Z, de Groot JR, Mulder BJ. Interventional and surgical treatment of cardiac arrhythmias in adults with congenital heart disease. Expert Rev Cardiovasc Ther 2010;8:1753-66. [Crossref] [PubMed]
- Khairy P, Ionescu-Ittu R, Mackie AS, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol 2010;56:1149-57. [Crossref] [PubMed]
- Bonchek LI, Burlingame MW, Worley SJ, et al. Cox/Maze procedure for atrial septal defect with atrial fibrillation: management strategies. Ann Thorac Surg 1993;55:607-10. [Crossref] [PubMed]
- Kobayashi J, Yamamoto F, Nakano K, et al. Maze procedure for atrial fibrillation associated with atrial septal defect. Circulation 1998;98:II399-402. [PubMed]
- Murphy JG, Gersh BJ, McGoon MD, et al. Long-term outcome after surgical repair of isolated atrial septal defect—follow-up at 27 and 32 years. New Engl J Med 1990;323:1645-50. [Crossref] [PubMed]
- Vecht JA, Saso S, Rao C, et al. Atrial septal defect closure is associated with a reduced prevalence of atrial tachyarrhythmia in the short to medium term: a systematic review and meta-analysis. Heart 2010;96:1789-97. [Crossref] [PubMed]
- Giamberti A, Chessa M, Foresti S, et al. Combined atrial septal defect surgical closure and irrigated radiofrequency ablation in adult patients. Ann Thorac Surg 2006;82:1327-31. [Crossref] [PubMed]
- Brown ML, Dearani JA, Danielson GK, et al. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. J Am Coll Cardiol 2008;52:460-6. [Crossref] [PubMed]
- Delhaas T, Sarvaas GJ, Rijlaarsdam ME, et al. A multicenter, long-term study on arrhythmias in children with Ebstein anomaly. Pediatr Cardiol 2010;31:229-33. [Crossref] [PubMed]
- Khairy P, Aboulhosn J, Gurvitz MZ, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation 2010;122:868-75. [Crossref] [PubMed]
- Backer CL, Tsao S, Deal BJ, et al. Maze procedure in single ventricle patients. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2008.44-8. [Crossref] [PubMed]
- Dearani JA, Mavroudis C, Quintessenza J, et al. Surgical advances in the treatment of adults with congenital heart disease. Curr Opin Pediatr 2009;21:565-72. [Crossref] [PubMed]
- Mavroudis C, Deal BJ, Backer CL, et al. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg 2007;84:1457-65; discussion 1465-6. [Crossref] [PubMed]
- Mavroudis C, Backer CL, Deal BJ, et al. Evolving anatomic and electrophysiologic considerations associated with Fontan conversion. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2007.136-45. [Crossref] [PubMed]
- Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease. Heart Rhythm 2014;11:e102-e165. [Crossref] [PubMed]
- Gillinov AM. Choice of surgical lesion set: answers from the data. Ann Thorac Surg 2007;84:1786-92. [Crossref] [PubMed]
- Cox JL, Jaquiss RD, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg 1995;110:485-95. [Crossref] [PubMed]
- Stulak JM, Dearani JA, Puga FJ, et al. Right-sided maze procedure for atrial tachyarrhythmias in congenital heart disease. Ann Thorac Surg 2006;81:1780-4; discussion 1784-5.
- Khargi K, Keyhan-Falsafi A, Hutten BA, et al. Surgical treatment of atrial fibrillation: a systematic review. Herzschrittmacherther Elektrophysiol 2007;18:68-76. [Crossref] [PubMed]
- Theodoro DA, Danielson GK, Porter CJ, et al. Right-sided maze procedure for right atrial arrhythmias in congenital heart disease. Ann Thorac Surg 1998;65:149-53; discussion 153-4. [Crossref] [PubMed]
- Sandoval N, Velasco VM, Orjuela H, et al. Concomitant mitral valve or atrial septal defect surgery and the modified Cox-maze procedure. Am J Cardiol 1996;77:591-96. [Crossref] [PubMed]
- Kant I. The only possible argument in support of a demonstration of the existence of God. In: Walford D, editor. Theoretical Philosophy, 1755-1770. The Cambridge Edition of the Works of Immanuel Kant. Cambridge: Cambridge University Press, 1992:107-201.
- Plato. The Republic. New York: Penguin Books, 1970.
- Aristotle. The Nicomachean ethics, book III. (written 350 B.C.E.). Ross D, trans. Oxford, England: Oxford University Press, 1980.
- Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. New Engl J Med 1999;340:839-46. [Crossref] [PubMed]
- Kamata J, Kawazoe K, Izumoto H, et al. Predictors of sinus rhythm restoration after Cox maze procedure concomitant with other cardiac operations. Ann Thorac Surg 1997;64:394-98. [Crossref] [PubMed]
- Cox JL, Jaquiss RD. Atrial septal defect. N Engl J Med 1996;334:57. [PubMed]
- Zomer AC, Vaartjes I, Uiterwaal CS, et al. Circumstances of death in adult congenital heart disease. Int J Cardiol 2012;154:168-72. [Crossref] [PubMed]
- Gallego P, Gonzalez AE, Sanchez-Recalde A, et al. Incidence and predictors of sudden cardiac arrest in adults with congenital heart defects repaired before adult life. Am J Cardiol 2012;110:109-17. [Crossref] [PubMed]
- Bouchardy J, Therrien J, Pilote L, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation 2009;120:1679-86. [Crossref] [PubMed]
- Mavroudis C, Backer CL, Deal BJ. Late reoperations for Fontan patients: state of the art invited review. Eur J Cardiothorac Surg 2008;34:1034-40. [Crossref] [PubMed]
- Van De Bruaene A, Delcroix M, Pasquet A, et al. The importance of pulmonary artery pressures on late atrial arrhythmia in transcatheter and surgically closed ASD type secundum. Int J Cardiol 2011;152:192-5. [Crossref] [PubMed]
- Stulak JM, Sharma V, Cannon BC. Optimal surgical ablation of atrial tachyarrhythmias during correction of Ebstein anomaly. Ann Thorac Surg 2015;99:1700-5. [Crossref] [PubMed]
- Deal BJ, Mavroudis C, Backer CL, et al. New directions in surgical therapy of arrhythmias. Pediatr Cardiol 2000;21:576-83. [Crossref] [PubMed]
- Deal BJ. Late arrhythmias following Fontan surgery. World J Pediatr Congenit Heart Surg 2012;3:194-200. [Crossref] [PubMed]
- d'Udekem Y, Iyengar AJ, Cochrane AD, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation 2007;116:I157-64. [Crossref] [PubMed]
- Yap SC, Harris L, Silversides CK, et al. Outcome of intra-atrial re-entrant tachycardia, catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol 2010;56:1589-96. [Crossref] [PubMed]
- Sealy WC, Hattler BG Jr, Blumenschein SD, et al. Surgical treatment of Wolff-Parkinson-White syndrome. Ann Thorac Surg 1969;8:1-11. [Crossref] [PubMed]
- Guiraudon GM, Klein GJ, Sharma AD, et al. Surgical treatment of supraventricular tachycardia: a five-year experience. Pacing Clin Electrophysiol 1986;9:1376-80. [Crossref] [PubMed]
- Mavroudis C, Deal BJ, Backer CL, et al. Arrhythmia surgery in patients with and without congenital heart disease. Ann Thorac Surg 2008;86:857-68; discussion 857-68. [Crossref] [PubMed]
- Mavroudis C, Deal BJ, Backer CL. Surgical therapy of cardiac arrhythmias. In: Mavroudis C, Backer CL, editors. Pediatric Cardiac Surgery, 4th ed. London, UK: Wiley Blackwell, 2013.
- Mavroudis C, Deal BJ, Backer CL, et al. Operative techniques in association with arrhythmia surgery in patients with congenital heart disease. World J Pediatr Congenit Heart Surg 2013;4:85-97. [Crossref] [PubMed]
- Karamlou T, Silber I, Lao R, et al. Outcomes after late reoperation in patients with repaired tetralogy of Fallot: the impact of arrhythmia and arrhythmia surgery. Ann Thorac Surg 2006;81:1786-93. [Crossref] [PubMed]
- Giamberti A, Chessa M, Abella R, et al. Surgical treatment of arrhythmias in adults with congenital heart defects. Int J Cardiol 2008;129:37-41. [Crossref] [PubMed]
- Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2016;67:e27-e115. [Crossref] [PubMed]
- Cox JL, Boineau JP, Schuessler RB, et al. Successful surgical treatment of atrial fibrillation. JAMA 1991;266:1976-80. [Crossref] [PubMed]
- Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg 1991;101:584-92. [PubMed]
- Cox JL, Schuessler RB, Lappas DG, et al. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg 1996;224:267-73, discussion 273-4. [Crossref] [PubMed]
- Cox JL, Boineau JP, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg 1995;110:473-84. [Crossref] [PubMed]
- Brodman RF, Frame R, Fisher JD, et al. Combined treatment of mitral stenosis and atrial fibrillation with valvuloplasty and a left atrial maze procedure. J Thorac Cardiovasc Surg 1994;107:622-4. [PubMed]
- Dewire J, Calkins H. Update on atrial fibrillation catheter ablation technologies and techniques. Nat Rev Cardiol 2013;10:599-612. [Crossref] [PubMed]
- Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822-8. [Crossref] [PubMed]
- Gaynor SL, Schuessler RB, Bailey MS, et al. Surgical treatment of atrial fibrillation: predictors of late recurrence. J Thorac Cardiovasc Surg 2005;129:104-11. [Crossref] [PubMed]
- Stulak JM, Dearani JA, Burkhart HM, et al. The surgical treatment of concomitant atrial arrhythmias during redo cardiac operations. Ann Thorac Surg 2012;94:1894-9. [Crossref] [PubMed]
- Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. J Thorac Cardiovasc Surg 2006;131:1029-35. [Crossref] [PubMed]
- Cosio FG. Understanding atrial arrhythmia mechanisms by mapping and ablation. Europace 2013;15:315-6. [Crossref] [PubMed]
- Wasmer K, Köbe J, Dechering DG, et al. Isthmus-dependent right atrial flutter as the leading cause of atrial tachycardias after surgical atrial septal defect repair. Int J Cardiol 2013;168:2447-52. [Crossref] [PubMed]
- Chan DP, Van Hare GF, Mackall JA, et al. Importance of atrial flutter isthmus in postoperative intra-atrial reentrant tachycardia. Circulation 2000;102:1283-9. [Crossref] [PubMed]
- Teh AW, Medi C, Lee G, et al. Long-term outcome following ablation of atrial flutter occurring late after atrial septal defect repair. PACE 2011;34:431-5. [Crossref] [PubMed]
- Lukac P, Pedersen AK, Mortensen PT, et al. Ablation of atrial tachycardia after surgery for congenital and acquired heart disease using an electroanatomic mapping system: Which circuits to expect in which substrate? Heart Rhythm 2005;2:64-72. [Crossref] [PubMed]
- Deal BJ, Mavroudis C, Backer CL, et al. Comparison of anatomic isthmus block with the modified right atrial maze procedure for late atrial tachycardia in Fontan patients. Circulation 2002;106:575-9. [Crossref] [PubMed]
- Mavroudis C. Arrhythmia surgery and pacemaker placement not associated with Fontan conversion. In: Mavroudis C, Backer CL, editors. Atlas of Pediatric Cardiac Surgery. London, UK: Springer Verlag, 2015.
- Gelatt M, Hamilton RM, McCrindle BW, et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol 1997;29:194-201. [Crossref] [PubMed]
- Khargi K, Hutten BA, Lemke B, et al. Surgical treatment of atrial fibrillation; a systemic review. Eur J Cardiothorac Surg 2005;27:258-65. [Crossref] [PubMed]
- Schuessler RB, Lee AM, Melby SJ, et al. Animal studies of epicardial atrial ablation. Heart Rhythm 2009;6:S41-5. [Crossref] [PubMed]
- Collins KK, Rhee EK, Delucca JM, et al. Modification to the Fontan procedure for the prophylaxis of intra-atrial reentrant tachycardia: short-term results of a prospective randomized blinded trial. J Thorac Cardiovasc Surg 2004;127:721-9. [Crossref] [PubMed]
- Geva T, Sandweiss BM, Gauvreau K, et al. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by manetic resonance imaging. J Am Coll Cardiol 2004;43:1068-74. [Crossref] [PubMed]
- Harrild DM, Berul CI, Cecchin F, et al. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation 2009;119:445-51. [Crossref] [PubMed]
- Deal BJ, Mavroudis C, Backer CL, et al. Impact of arrhythmia circuit cryoablation during Fontan conversion for refractory atrial tachycardia. Am J Cardiol 1999;83:563-8. [Crossref] [PubMed]
- Mavroudis C, Backer CL, Deal BJ, et al. Total cavopulmonary conversion and maze procedure for patients with failure of the Fontan operation. J Thorac Cardiovasc Surg 2001;122:863-71. [Crossref] [PubMed]
- Mavroudis C, Deal BJ, Backer CL. Surgery for arrhythmias in children. Int J Cardiol 2004;97:39-51. [Crossref] [PubMed]


