Obstetrical, maternal characteristics and outcome of HIV-infected rapid progressor infants at Yaounde: a retrospective study
Introduction
The proportion of HIV-infected who are rapid progressor has decreased with the improvement of the quality of Prevention of Mother to Child Transmission (PMTCT), and more specifically with early management of the mother and child (1). In 2011, the demographic health survey conducted in Cameroon showed that the HIV seroprevalence in women aged 15–49 years who were pregnant during the survey was 5.6% (2). However, the performance level of the PMTCT program is rather low nationwide, with only 21.4% of the HIV infected pregnant women receiving anti-retroviral (ARV) prophylaxis (3). Many HIV exposed infants born to mothers who have received PMTCT interventions may have missed out some of the interventions that target infants. In fact, about 74.3% of infants did not receive any ARV prophylaxis according to the report of the ministry of public health (3). A study carried out in the Eastern Region of Cameroon showed that 65.2% of exposed infants and 91.1% of mothers did not receive any ARV prophylaxis for PMTCT (4).
Pediatric HIV infection is most often transmitted vertically, either during pregnancy, delivery or during breastfeeding (5-10). Newell et al. found that without any intervention, 35.2% of HIV infected children will die at the end of the first year and 52.5% by two years in resource limited countries (11). Before that Blanche et al. showed that 44% of HIV-1 infected infants born to mothers with severe disease at delivery, will die during the first 18 months of life as a result of HIV encephalopathy and opportunistic infections (12). Rapid progressors are infants who develop severe signs of AIDS very early in life usually leading to death for most of them (13,14). Many factors are described in literature concerning HIV-infected rapid progressors (15-18). It has been proven that there is a relationship between high maternal viral load and severe immunodeficiency, and high viral load in infants (15,19-22). The severity of immunosuppression, and high viral loads in infants (23-25) are associated with rapid progression of HIV infection. On the contrary, maternal treatment even with prophylactic regimen, would indirectly reduce the progression of the disease in the infant (15,16,19,26). Some associated factors could be found in our milieu because of concurrent illness. The objective of our study was to explore factors which can help in diagnosing rapid progressor children and their outcome.
Methods
Study framework
A retrospective study was carried out to identify rapid progressor infants seen between January 2010 and February 2012 at the Mother and Child Centre of the Chantal Biya Foundation (CME-CBF). The CME-CBF is a pediatric centre which handles the largest cohort of children on ARVs in Cameroon. HIV testing of children less than 18 months was done using DNA/RNA PCR. The PCR was done at the Centre Pasteur du Cameroun in the framework of the ANRS Pediacam study (27) and at the Chantal Biya International Research Center (CBIRC) (28). The children were referred to the CME-FCB either for diagnosis of HIV in those suspected to be infected or for further management of the already diagnosed HIV infection. The others were HIV exposed infants followed up in PMTCT. Patients are managed at the study site following the national guidelines for HIV exposed and infected children (29).
Study population
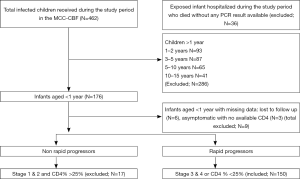
HIV-infected infants less than 12 months old received and followed up at the HIV treatment centere of the CME-FCB were included in this study. We excluded asymptomatic HIV-infected subjects with no available CD4 count and also exposed infants of HIV-infected mothers without PCR result.
Procedure and data collection
We reviewed the medical records of the HIV-infected children from the database of the site. According to our inclusion criteria, we selected those in whom the signs and symptoms corresponded to stage 4 of the WHO classification or who had CD4 counts below 25% no matter their clinical stage, whether they had been on ARV therapy or not. Mothers or guardians who accompanied their infants to routine consultations were interviewed on the pregnancy that led to the birth of the child. Data which could not be obtained from mothers’ interview (mothers who died or were absent) were collected from the medical records of the patients. Reasons why PMTCT was not carried out were sought. We collected socio-demographic data of the mothers such as: age, level of education, social status. We then obtained information concerning the maternal obstetrical history. This was the number of antenatal clinics (ANCs) attended, the existence of any pathologies during the pregnancy and PMTCT measures carried out, as well as the perinatal history. We also found out about cases of death of siblings.
Concerning the infants, we recorded their age, sex, immunization status, the psychomotor development, comorbid conditions and prior hospitalisations. We also evaluated their nutritional status and found out whether they received Cotrimoxazole and ARV prophylaxis, feeding practices, biological analysis (CD4 count, and viral load).
Main outcome and other variables definitions
“Rapid progressor infant” was defined as any HIV-infected infant whether on HAART or not who developed during his first year of life, a sign which classifies him in clinical stage 3 or 4 or a severe immunodeficiency (% CD4 <25) or both (30). Patients survival during the study period was evaluated. We then estimated their probability of survival with or without ARV treatment from birth to the end of the study using the Kaplan-Meier method.
Other variables as mentioned above were used. The feeding method were defined as; infant formula feeding, breastfeeding or mixed when other food, solid or liquid was given in addition to breast milk. Protected breastfeeding was described when the mother was receiving or had received prophylactic ARVs and as well as the infant, for up to one week following the end of the breastfeeding. Their clinical and immunological stages were determined using the WHO classification. Infants were divided into two groups; minor immunodeficiency (those in stage 1 and 2), and major immunodeficiency (those in stages 3 and 4).
Statistical analysis
Data was analysed using the Epi Info 7 software and Microsoft Excel 2010. Through the univariate analysis of maternal and infant characteristics, we described the categorical variables in frequencies and percentages. Medians and interquartile ranges were used for continuous variables. Bivariate analysis consisted of estimating the odds ratio measuring the obstetrical, mothers and infants characteristics of the HIV-infected rapid progressors within the 95% confidence intervals. We use the Log-rank test to calculate P value which we analysed. P values <0.05 were considered statistically significant.
Ethical considerations
Our study was approved by the Institutional Ethics and Research Committee of the Faculty of Medicine and Biomedical Sciences of the University of Yaounde I. A written or verbal informed consent was obtained from parents or guardians prior to their interview and data collection. Both clinical records and laboratory results were kept confidential.
Results
Socio-demographic data
In all, 462 HIV-1-infected children were received on site during the study period; no case of HIV-2 was recorded. Among the subjects, we identified 176 HIV-1 infected infants aged less than 12 months. Of the 150/(32.5%) HIV-infected children who fulfilled our inclusion criteria, 85.2% of them were rapid progressors (Figure 1). The median age was 6 months with an interquartile range [4; 10 months] during the enrolment. Of the 150 rapid progressors, 19 (12.7%) had lost their mothers; 13 (8.7%) their fathers and 4 (2.7%) both parents when the diagnosis of HIV infection was made. At enrolment, 10.7% were accompanied by a guardian. Mean age of the living mothers was 27 years, ranging from 18 to 43 years, 45.4% were single or 42.0% were cohabiting (not officially married). Almost all of them 97.7% (128/131) were literate with 61.0% having attained secondary school level and half (45.8%) were housewives (Table 1). Most of the children who were rapid progressor 80.7% (121/150) had siblings with 50.7% of them having more than 3 siblings. In 11.3% of cases, there was a history of death of a sibling occurring between the ages of 3 to 16 months with a median age of 8 months; their HIV status as well as that of their mothers were unknown at the time of death.

Full table
Antenatal history and prevention of mother to child transmission of HIV (PMTCT)
Most of the mothers interviewed (65.4%) started ANC during the second trimester, others either during the first (11.3%) or the third (17.3%) trimester, while some (6%) never attended ANC. About 47.2% (62/131) of mothers were hospitalized during their pregnancy because of one or a combination of pathologies including gastro-enteritis (59.7%), pulmonary infections (48.4%), malaria (43.5%); genital and urinary tract infections (20.9%) and pulmonary tuberculosis (19.4%). PMTCT measures were not carried out in 2/3 of these mothers, 105 pregnant women (70.0%) did not take any ARV prophylaxis. Most of them did not know their HIV status. The status for 80 (53.3%) of them was discovered after delivery, and for others when the child was ill. Twenty three mothers (15.3%) knew their HIV status before pregnancy, 35 (23.3%) during pregnancy with 2%, 14.7% and 8.7% during the first, the second and the third trimester respectively. Nine (6.0%) were informed at delivery (Table 1). Ignorance of their HIV status, denial of the test results and no ARVs given by health care providers were some of the reasons given for the absence of ARV prophylaxis. Of the 45 mothers who received ARVs, 15 (33.3%) were on HAART, 9 of whom started ARV before; 5 during pregnancy and 1 after childbirth. Others 30/45 (66.7%) received ARV prophylaxis, 10 (33.3%) of whom received single dose Nevirapine during labour. Some mothers, 19 (12.7%) gave birth at home. Only 41 (27.3%) CD4 counts performed during pregnancy were reported and these showed a severe immunodeficiency with CD4 count <200/mm3 for 12 of them (29.3%).
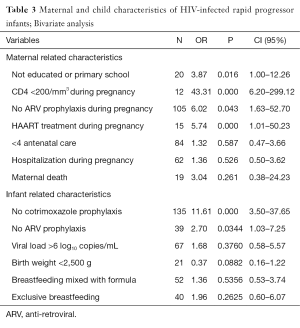
Significant maternal factors associated with subjects were the low level of education (OR=3.87; P=0.016), CD4 count less than 200/mm3 during pregnancy (OR=43.3; P=0.000), absence of ARV prophylaxis (OR=6.02; P=0.043), or treatment with HAART (OR=5.74; P=0.000).
Characteristics of the rapid progressor infants
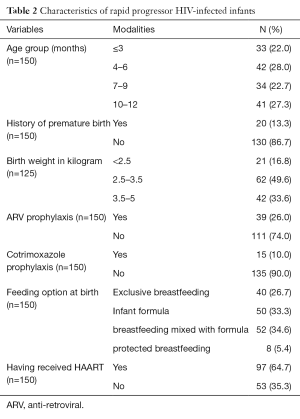
At enrolment, 87 infants (58.0%) were in clinical stage 4 while the others were selected based on severe immune deficiency. The viral load was available only in 79 (52.7%) infants and the values ranged between 111.3 and 663 log10 copies/mL with an average of 87.7 log10 copies/mL. Only 39 rapid progressors (26.0%) had received ARV prophylaxis at birth (Table 2), more than 3/4 (76.9%) of whom had been on bitherapy with Zidovudine and Nevirapine, the rest received monotherapy; 12.8% received Nevirapine and 10.7% Zidovudine.
Full table
In infants, the most significant events were the absence of Cotrimoxazole prophylaxis (OR=11.61; P=0.000) and antiretroviral prophylaxis (OR=2.70; P=0.0344); (Table 3).
Full table
Outcome of the rapid progressors
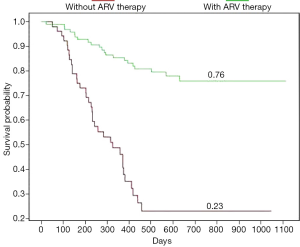
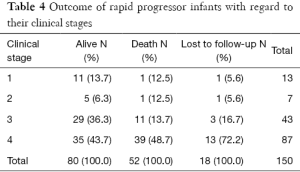
Death occurred in 52/150 (34.7%) of the patients during the study period, at a median age of 7 months (Table 4). The survival rate was higher (84.3%) in infants who were receiving ARV treatment (P<0.05) as opposed to 43.3% in those who were not (Figure 2).
Full table
Discussion
This retrospective study described the HIV-infected rapid progressors with regards to the mothers’ obstetrical characteristics and those of the children. The proportion of the rapid progressor was high in our study. Our study has not been performed on a cohort of infant. We recruited at a specialized HIV/AIDS unit where most of the children were symptomatic at referral. Most of them came either from our admission units or from others health facilities for confirmation of their HIV status or for follow up. Some were HIV exposed infants followed in the PMTCT unit. These could have introduced some bias.
We found patients at clinical stage 1 & 2 with a severe immunological deficiency (CD4 count <25%). Some had severe clinical stage but no profound immune-depression. We also described the socio-demographic background of the mother and infant couple and found out whether they benefited from PMTCT interventions. Only 12.2% of the mothers were employed. The socio-demographic conditions were poor for many; in fact, 12.7%, 8.7% and 2.7% of infants had lost their mothers, fathers or both parents respectively. Such conditions increase the vulnerability of the subjects to illnesses and death (31). The maternal factors that were significantly associated with advanced WHO clinical stage of the rapid progressors were a CD4 count <200/mm3, absence of ARVs prophylaxis or not being on HAART. Previous studies have shown that severe immune deficiency in mothers was associated with early poor progression of the HIV in infants (16,32). The same would be true with a high maternal viral load during delivery (33,34). Ideally, testing of women of child-bearing age and pregnant women could permit early HIV screening and the initiation of ARV treatment or the PMTCT program in case of infection. Early detection of HIV infection in mothers as from the 14th week of gestation and of every exposed child at birth are cost-effective (35). In our study, 65.4% of the pregnant women started ANC during the second trimester and 53.3% had their HIV screening performed after delivery and for some when the children were ill. Hence, most (70.0%) of them did not receive any ARV prophylaxis during pregnancy meanwhile its effectiveness has been proven in the reduction of the rate of rapid disease progression in children (15,16,19,26). Thus, the rapid progressors seem to be particularly associated with such situations in our study (OR=6.02; P=0.043). It has been demonstrated that HAART is more effective in the prevention of severe clinical forms in children than ARV prophylaxis (11,15). In our population however, the benefits of HAART during pregnancy was not remarkably associated with rapid progressor infants. Only 15 women received HAART during pregnancy (Table 1).We did not investigate the existence of a relationship between the onset of treatment and the gestational age. One could imagine cases in which the HAART had not yet induced significant effects on the immunity of some mothers, particularly those who were severely immuno-compromised during the pregnancy. Analysis of rapid progressors’ specific conditions revealed an association between the absence of prophylaxis with cotrimoxazole (P=0.000, OR=11.61), or ARVs (P=0.0344, OR=2.70), and the severity of the clinical and immunological presentation in this study. Indeed, cotrimoxazole can prevent under certain conditions, severe infections in infants infected with HIV. It can reduce mortality by 43% and hospitalizations by 23% (36,37).
The mortality rate was higher (34.7%) in our population (Table 4), compared to that found in Nepal (38). It others setting very high mortality rate where found in infants who were not on ARV compared to those receiving ARV treatment (14). Death occurred in our study at a median age of 7 months; HIV infection increased the death rate before the age of 2 years considerably (39,40). The survival rate was low (43.3%) in children who were not treated, against 84.3% in those on ARV treatment. It has been demonstrated that HAART increases survival in approximately 95% of patients; meanwhile, death is almost inevitable for those without treatment (41). For this reason, authors suggested that HAART should be given as early as possible for all HIV-infected children (1,13,42).
Conclusions
In our study, mothers were often not aware of their HIV status and some did not have access to PMTCT services. According to authors, such mothers constitute a target population, for whom early diagnosis of their children is important (43). HAART before pregnancy for women eligible to treatment would improve their CD4 count before pregnancy. It must be associated to cotrimoxazole and ART prophylaxis in infants, as well as HAART in infant with a positive PCR in order to improve their survival rate.
Acknowledgements
The authors thank all the staff of the CME-CBF HIV management unit for their help, and the laboratories of the CPC and CBIRC as well for their collaboration. They also wish to thank Mr Frederic Tchuisseu, the statistician and Dr Fru Soh Florence, the translator of the document from French to English.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: For each patient, parent’s or guardian’s written or the verbal consent was obtained prior to the enrolment of their infant.
References
- Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359:2233-44. [Crossref] [PubMed]
- CAMEROUN - Enquête Démographique et de Santé et l’Enquête par grappe à Indicateurs Multiples (2011), Quatrième série des EDS et des MICS. 2011. Available online: http://nada.stat.cm/index.php/catalog/34
- Ministry of Public Health-Cameroon. Vers l’élimination virtuelle de la transmission du VIH de la mère à l’enfant à l’horizon 2015, rapport de progrès numéro 6, année 2011’, Rapport de progrès, 2012.
- Noubiap JJ, Bongoe A, Demanou SA. Mother-to-child transmission of HIV: findings from an early infant diagnosis program in Bertoua, Eastern Cameroon. Pan Afr Med J 2013;15:65. [PubMed]
- Jourdain G, Mary JY, Coeur SL, et al. Risk factors for in utero or intrapartum mother-to-child transmission of human immunodeficiency virus type 1 in Thailand. J Infect Dis 2007;196:1629-36. [Crossref] [PubMed]
- Al-Husaini AM. Role of placenta in the vertical transmission of human immunodeficiency virus. J Perinatol 2009;29:331-6. [Crossref] [PubMed]
- Humphrey JH, Marinda E, Mutasa K, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ 2010;341:c6580. [Crossref] [PubMed]
- Kourtis AP, Ibegbu CC, Wiener J, et al. Role of intestinal mucosal integrity in HIV transmission to infants through breast-feeding: the BAN study. J Infect Dis 2013;208:653-61. [Crossref] [PubMed]
- Selvaraj S, Paintsil E. Virologic and host risk factors for mother-to-child transmission of HIV. Curr HIV Res 2013;11:93-101. [Crossref] [PubMed]
- Chaillon A, Samleerat T, Zoveda F, et al. Estimating the timing of mother-to-child transmission of the human immunodeficiency virus type 1 using a viral molecular evolution model. PLoS One 2014;9:e90421. [Crossref] [PubMed]
- Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004;364:1236-43. [Crossref] [PubMed]
- Blanche S, Mayaux MJ, Rouzioux C, et al. Relation of the course of HIV infection in children to the severity of the disease in their mothers at delivery. N Engl J Med 1994;330:308-12. [Crossref] [PubMed]
- Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One 2012;7:e28510. [Crossref] [PubMed]
- Desmonde S, Coffie P, Aka E, et al. Severe morbidity and mortality in untreated HIV-infected children in a paediatric care programme in Abidjan, Côte d'Ivoire, 2004-2009. BMC Infect Dis 2011;11:182. [Crossref] [PubMed]
- Abrams EJ, Wiener J, Carter R, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. AIDS 2003;17:867-77. [Crossref] [PubMed]
- Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 2007;26:519-26. [Crossref] [PubMed]
- Huang S, Dunkley-Thompson J, Tang Y, et al. Deficiency of HIV-Gag-specific T cells in early childhood correlates with poor viral containment. J Immunol 2008;181:8103-11. [Crossref] [PubMed]
- Ciaranello A, Lu Z, Ayaya S, et al. Incidence of World Health Organization stage 3 and 4 events, tuberculosis and mortality in untreated, HIV-infected children enrolling in care before 1 year of age: an IeDEA (International Epidemiologic Databases To Evaluate AIDS) East Africa regional analysis. Pediatr Infect Dis J 2014;33:623-9. [Crossref] [PubMed]
- Mphatswe W, Blanckenberg N, Tudor-Williams G, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS 2007;21:1253-61. [Crossref] [PubMed]
- Mutasa K, Ntozini R, Prendergast A, et al. Impact of six-week viral load on mortality in HIV-infected Zimbabwean infants. Pediatr Infect Dis J 2012;31:948-50. [Crossref] [PubMed]
- Whitmore SK, Taylor AW, Espinoza L, et al. Correlates of mother-to-child transmission of HIV in the United States and Puerto Rico. Pediatrics 2012;129:e74-81. [Crossref] [PubMed]
- Gumbo FZ, Kandawasvika GQ, Kurewa EN, et al. Survival of HIV Infected Children Born to Mothers Enrolled in a PMTCT Program in a Resource Poor Setting. World Journal of AIDS 2013;3:119-23. [Crossref]
- Rich KC, Fowler MG, Mofenson LM, et al. Maternal and infant factors predicting disease progression in human immunodeficiency virus type 1-infected infants. Women and Infants Transmission Study Group. Pediatrics 2000;105:e8. [Crossref] [PubMed]
- Obimbo EM, Mbori-Ngacha DA, Ochieng JO, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected african children. Pediatr Infect Dis J 2004;23:536-43. [Crossref] [PubMed]
- Duri K, Gumbo FZ, Kristiansen KI, et al. Antenatal HIV-1 RNA load and timing of mother to child transmission; a nested case-control study in a resource poor setting. Virol J 2010;7:176. [Crossref] [PubMed]
- Mahdavi S, Malyuta R, Semenenko I, et al. Treatment and disease progression in a birth cohort of vertically HIV-1 infected children in Ukraine. BMC Pediatr 2010;10:85. [PubMed]
- Tejiokem MC, Faye A, Penda IC, et al. Feasibility of early infant diagnosis of HIV in resource-limited settings: the ANRS 12140-PEDIACAM study in Cameroon. PLoS One 2011;6:e21840. [Crossref] [PubMed]
- Nkenfou CN, Lobé EE, Ouwe-Missi-Oukem-Boyer O, et al. Implementation of HIV early infant diagnosis and HIV type 1 RNA viral load determination on dried blood spots in Cameroon: challenges and propositions. AIDS Res Hum Retroviruses 2012;28:176-81. [Crossref] [PubMed]
- Ministry of Public Health-Cameroon. Guide pour la prise en charge des enfants exposés et infectés par le VIH/SIDA 2012. Available online: www.emtct-iatt.org/../Cameroon_National-Pediatric-ARV-Guidelines_20
- Chearskul S, Chotpitayasunondh T, Simonds RJ, et al. Survival, disease manifestations, and early predictors of disease progression among children with perinatal human immunodeficiency virus infection in Thailand. Pediatrics 2002;110:e25. [Crossref] [PubMed]
- Bucagu M, Bizimana Jde D, Muganda J, et al. Socio-economic, clinical and biological risk factors for mother - to - child transmission of HIV-1 in Muhima health centre (Rwanda): a prospective cohort study. Arch Public Health 2013;71:4. [Crossref] [PubMed]
- Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis 2005;41:1654-61. [Crossref] [PubMed]
- Rouet F, Sakarovitch C, Msellati P, et al. Pediatric viral human immunodeficiency virus type 1 RNA levels, timing of infection, and disease progression in African HIV-1-infected children. Pediatrics 2003;112:e289. [Crossref] [PubMed]
- Ioannidis JP, Tatsioni A, Abrams EJ, et al. Maternal viral load and rate of disease progression among vertically HIV-1-infected children: an international meta-analysis. AIDS 2004;18:99-108. [Crossref] [PubMed]
- Udeh B, Udeh C, Graves N. Perinatal HIV transmission and the cost-effectiveness of screening at 14 weeks gestation, at the onset of labour and the rapid testing of infants. BMC Infect Dis 2008;8:174. [Crossref] [PubMed]
- Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet 2004;364:1865-71. [Crossref] [PubMed]
- Harambat J, Fassinou P, Becquet R, et al. 18-month occurrence of severe events among early diagnosed HIV-infected children before antiretroviral therapy in Abidjan, Côte d'Ivoire: a cohort study. BMC Public Health 2008;8:169. [Crossref] [PubMed]
- Diese M, Shrestha L, Pradhan B, et al. Bottlenecks and opportunities for delivering integrated pediatric HIV services in Nepal. Curr Opin HIV AIDS 2016;11:S21-9. [Crossref] [PubMed]
- Little K, Thorne C, Luo C, et al. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Curr HIV Res 2007;5:139-53. [Crossref] [PubMed]
- Brahmbhatt H, Kigozi G, Wabwire-Mangen F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr 2006;41:504-8. [Crossref] [PubMed]
- Evans-Gilbert T, Pierre RB, Steel-Duncan J, et al. HIV-related mortality in Jamaican children. West Indian Med J 2008;57:265-8. [PubMed]
- Ndondoki C, Dabis F, Namale L, et al. Survival, clinical and biological outcomes of HIV-infected children treated by antiretroviral therapy in Africa: systematic review, 2004-2009. Presse Med 2011;40:e338-57. [Crossref] [PubMed]
- Ciaranello AL, Park JE, Ramirez-Avila L, et al. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med 2011;9:59. [Crossref] [PubMed]